Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Method Article
Augenstielablation zur Steigerung der Ovarialreifung bei Schlammkrabben
In diesem Artikel
Erratum Notice
Zusammenfassung
Zwei Protokolle zur Ablation des Augenstiels (d. h. Kauterisations- und Operationsansätze) wurden an anästhesierten weiblichen Krabben durchgeführt. Die Augenstielablation von Schlammkrabben beschleunigte die Reifung der Eierstöcke, ohne die Überlebensrate zu verringern.
Zusammenfassung
Schlammkrabben (Scylla spp.) sind kommerziell wichtige Krebstierarten, die im gesamten indo-westpazifischen Raum vorkommen. Während der Kultur ist die Induktion der Eierstockreifung wichtig, um die Nachfrage der Verbraucher nach reifen Schlammkrabben zu befriedigen und die Samenproduktion zu beschleunigen. Die Augenstielablation ist ein wirksames Mittel, um die Reifung der Eierstöcke bei Schlammkrabben zu verbessern. Es gibt jedoch kein Standardprotokoll für die Augenstielablation von Schlammkrabben. In dieser Studie werden zwei Techniken zur Ablation des Augenstiels beschrieben: die Kauterisation (die Verwendung von heißem Metall zur Ablation des Augenstiels einer anästhesierten Krabbe) und die Operation (die Entfernung des Augenstiels mit einer chirurgischen Schere). Vor der Augenstielablation wurden geschlechtsreife Weibchen (CW > 86 mm) mit einem Eisbeutel (−20 °C) mit Meerwasser betäubt. Als die Wassertemperatur 4 °C erreichte, wurde der Eisbeutel aus dem Wasser genommen. Fließendes Meerwasser (Umgebungstemperatur: 28 °C) wurde zur Erholung von der Narkose unmittelbar nach der Augenstielablation verwendet. Eine Mortalität trat weder während noch nach dem Prozess der Augenstielablation auf. Das hier vorgestellte Protokoll zur Ablation des Augenstiels beschleunigte die ovarielle Reifung der Schlammkrabben.
Einleitung
Alle vier Schlammkrabbenarten der Gattung Scylla sind kommerziell wichtige Krebstierarten in der Aquakultur 1,2. Das Wachstum von Krebstieren, einschließlich Schlammkrabben, und ihre Umwandlung von der vorzeitigen (subadulten oder pubertären) Phase in die geschlechtsreife (adulte) Phase erfolgt durch einen Häutungsprozess, der das periodische Abwerfen älterer und kleinerer Exoskelette beinhaltet. Die Panzerbreite (CW), die Morphologien der Chelipeden und der Bauchlappen werden häufig verwendet, um die Geschlechtsreife von Scylla spp. zu bestimmen. 3,4,5. Der Prozess der Häutung wird durch die Wirkung verschiedener Hormone reguliert und erfordert eine große Menge an Energie6. Zusätzlich zum normalen Häutungsprozess beschleunigt der Verlust von Gliedmaßen, entweder freiwillig oder durch äußere Faktoren verursacht, die Häutung der Krabben, ohne ihre Überlebensrate zu beeinträchtigen 7,8,9. Daher wird die Autotomie der Gliedmaßen häufig zur Häutungsinduktion in der Weichschalen-Schlammkrabbenzuchtindustrie eingesetzt 7,9.
Die einseitige oder beidseitige Ablation des Augenstiels ist vor allem bei Süßwassergarnelen und Meeresgarnelen für die Reifung der Keimdrüsen und die Samenproduktion beliebt10,11,12,13. Gängige Techniken zur Ablation des Augenstiels bei Krebstieren sind die folgenden: (i) Ligatur an der Basis des Augenstiels mit einer Schnur14,15; ii) Verätzung des Augenstiels mit einer heißen Pinzette oder Elektrokauterisationsvorrichtung16; iii) Entfernen oder direktes Einklemmen des Augenstiels, um eine offene Wunde12 zu hinterlassen; und (iv) Entfernung des Inhalts des Augenstiels durch Inzision nach dem Aufschneiden des distalen Teils des Auges mit einem Rasierer17. Die X-Organe des Augenstiels sind wichtige endokrine Organe bei Krebstieren, da sie hyperglykämische Hormone (CHH), häutungshemmende Hormone (MIH) und vitellogenesehemmende Hormone (VIH) regulieren6,18,19,20,21,22. Die X-Organe des Augenstiels (oder der Sinusdrüsenkomplex) synthetisieren und setzen Gonaden-hemmende Hormone (GIH) frei, die auch als vitellogenesehemmende Hormone (VIH) bekannt sind und zur Familie der Neuropeptidhormonegehören 6. Die einseitige oder beidseitige Ablation des Augenstiels reduziert die GIH-Synthese, was zur Dominanz stimulierender Hormone (d. h. Gonaden-stimulierende Hormone, GSH) und zur Beschleunigung des ovariellen Reifungsprozesses bei Krebstieren führt23,24,25,26. Ohne den Einfluss von GIH nach der Ablation des Augenstiels widmen weibliche Krebstiere ihre Energie der Entwicklung der Eierstöcke27. Es wurde festgestellt, dass eine einseitige Augenstielablation für die Induktion der Ovarialreifung bei Krebstierenausreicht 11 und dass sich der abgetragene Augenstiel von Garnelen und Krebsen nach mehreren Häutungen regenerieren kann28. Bei Scylla spp. sind vier ovarielle Entwicklungsstadien verzeichnet: i) unreif (Stadium-1), ii) frühreif (Stadium-2), iii) vorzeitig reifend (Stadium-3) und iv) voll ausgereift (Stadium-4)29,30. Das unreife Ovarialstadium findet sich bei unreifen Frauen. Nach der Pubertätshäutung und Paarung beginnt sich der unreife Eierstock zu entwickeln und reift schließlich (Stadium 4), bevor er31 laicht.
Ein Augenstiel-Ablationsprotokoll ist für die Entwicklung des Brutbestands und die Samenproduktion von Schlammkrabben unerlässlich. Auf dem globalen Lebensmittelmarkt werden ausgewachsene Schlammkrabben mit voll ausgereiften Eierstöcken (Stadium 4) gegenüber Krabben mit höherem Muskelanteil von den Verbrauchern bevorzugt und haben daher einen höheren kommerziellen Wert, sogar höher als große Männchen. Es gibt kein vollständiges Protokoll für die Augenstielablation von Schlammkrabben. Das Augenstielablationsprotokoll in dieser Arbeit minimiert Stress durch die Verwendung vollständig betäubter Krabben und minimiert körperliche Verletzungen des Personals durch Krabbenbisse. Dieses Protokoll ist einfach und kostengünstig. In dieser Arbeit stellen wir ein Protokoll für die Augenstielablation von Scylla spp. vor, das die Reifung der Keimdrüse induzieren kann. Zwei Techniken der Augenstielablation (Kauterisation und Operation) wurden getestet und ihre Effizienz anhand der Gonadenentwicklungsrate weiblicher Schlammkrabben verglichen.
Protokoll
Dieses Protokoll folgt dem malaysischen Verhaltenskodex für die Pflege und Verwendung von Tieren für wissenschaftliche Zwecke, der von der Laboratory Animal Science Association of Malaysia festgelegt wurde. Die Opferung der Versuchsproben erfolgte gemäß dem National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, überarbeitet 1978). Geschlechts vorzeitige weibliche Schlammkrabben (orangefarbene Schlammkrabbe S. olivacea) wurden auf dem lokalen Markt (5°66′62′′N, 102°72′33′′E) in den Setiu-Feuchtgebieten in Malaysia gesammelt. Die Schlammkrabbenart wurde anhand morphologischer Merkmaleidentifiziert 1.
1. Probenentnahme und Desinfektion
- Sammeln Sie gesunde, aktive und vorzeitig ausgewachsene weibliche Schlammkrabben (Abbildung 1).
HINWEIS: Vorzeitige weibliche Krabben haben dreieckige und helle Hinterleibslappen sowie einen CW-Bereich von 80-85 mm. - Waschen Sie die Krabben mit gechlortem Leitungswasser (Süßwasser), um Schmutz und osmophile Parasiten zu entfernen.
- Die Krabben 30 Minuten lang in 150 ppm Formaldehyd mit 20 ppt Salzgehalt einweichen.
- Sorgen Sie während der Formaldehydbehandlung für eine kontinuierliche und schonende Belüftung mit Ausströmern. Die Belüftungsquelle kann entweder von einer zentralen Belüftungsleitung oder einer Aquarienbelüftungspumpe stammen.
- Waschen Sie die Krabben mit fließendem Meerwasser, um Formaldehydreste zu entfernen.
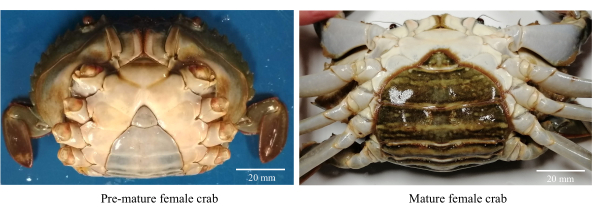
Abbildung 1: Abdominale Morphologie weiblicher Schlammkrabben zur Identifizierung der sexuellen Reifungsstadien. Bitte klicken Sie hier, um eine größere Version dieser Abbildung zu sehen.
2. Akklimatisierung
- Jede desinfizierte Hündin wird in einen separaten runden 32-Liter-Tank umgefüllt.
- Ziehen Sie die Weibchen 3 Tage lang bei einem Salzgehalt von 20 ppt auf und füttern Sie sie zweimal täglich (morgens 09:00 Uhr und abends 20:00 Uhr) mit gehacktem Meeresfisch mit etwa 4 % bis 5 % des Körpergewichts der Krabbe.
- Entfernen Sie überschüssiges und nicht gefressenes Futter, indem Sie es vor der morgendlichen Fütterung absaugen.
- Tauschen Sie täglich 10 % des Meerwassers der Krabbenaufzucht (20 ppt) aus.
3. Induzierte Häutung für die Geschlechtsreife
- Schneiden Sie alle Beine außer den Schwimmbeinen mit einer sterilisierten Schere ab.
- Fangen Sie die Krabbe mit einem Schöpfnetz und halten Sie die Krabbe vorsichtig fest. Schneiden Sie zuerst beide Chelipeds und dann die Laufbeine am zweiten Gelenk mit einer Schere ab. Die Krabbe autotomisiert die beschädigten Gliedmaßen automatisch. Eine Anästhesie ist für die Autotomie der Gliedmaßen nicht erforderlich.
- Waschen Sie die Krabbe sofort nach der Autotomie der Gliedmaßen in Süßwasser.
- Übertragen Sie die gliedmaßenautotomisierten Krabben einzeln in perforierte Kunststoffkörbe (28 cm L x 22 cm B x 7 cm H) und legen Sie sie in ein Fiberglasbecken (305 cm L x 120 cm B x 60 cm H).
Anmerkungen: Zwei Körbe können zusammengebunden und zusammengeklipst werden. Der obere Korb dient als Abdeckung, damit die Krabbe nicht aus dem Korb entkommen kann. - Verwenden Sie ein Aquakultur-Kreislaufsystem (RAS) mit einem Salzgehalt von 20 ppt und einer Wassertiefe von mindestens 10 cm, um sicherzustellen, dass der gesamte Kunststoffkorb untergetaucht ist.
- Füttern Sie die weibliche Krabbe zweimal täglich mit gehacktem Meeresfisch mit 5 % bis 7 % des Körpergewichts der Krabbe.
- Aufziehen der Krabben bis zur Geschlechtsreife durch Häutung (35 Tage).
HINWEIS: Die induzierte Häutung kann für die kommerzielle Reifung der Eierstöcke und die Samenproduktion mit wilden, reifen weiblichen Schlammkrabben übersprungen werden. Geerntete, ausgewachsene Weibchen aus der Wildnis müssen akklimatisiert und direkt einer Kälteschockanästhesie und anschließender Augenstielablation unterzogen werden.
4. Anästhesie
- Selektieren Sie geschlechtsreife Weibchen mit einem dunkel gefärbten, ovalen Bauchlappen mit einem CW >86 mm (Abbildung 1).
- Fangen Sie die Krabben mit einem Schöpfnetz und halten Sie sie einzeln in kleinen Aquarien zur Narkose.
- Nach 5 Minuten Akklimatisierungszeit 2-Phenoxyethanol (2-PE) mit 2 ml/l in jedes Aquarium geben und 15 Minuten Narkosebehandlung einwirken lassen.
- Stellen Sie sicher, dass die Krabben durch das Fehlen spontaner Bewegungen vollständig betäubt sind.
5. Ablation des Augenstiels
- Kauterisationstechnik
- Führen Sie alle Vorgänge auf einem Tisch und in einem offenen Bereich durch.
- Nehmen Sie einen flachen Nickelstahl-Metallstab (z. B. einen Schraubendreher) mit einem Holz- oder Kunststoffgriff und decken Sie den Griff mit einem feuchten Baumwolltuch ab.
- Sterilisieren Sie zwei rostfreie chirurgische Pinzetten in einem Autoklaven.
- Bereiten Sie 70%iges Ethanol in einer Sprühflasche vor und halten Sie es von feuergefährdenden Quellen wie Lötlampe und glühendem Schraubendreher fern. Halten Sie Seidenpapier bereit.
Anmerkungen: Ethanol ist leicht entzündlich. Halten Sie einen Sicherheitsabstand zu Feuerquellen ein. - Schließen Sie eine Lötlampe sicher an eine Gasflasche (Butan) an.
VORSICHT: Befolgen Sie die Anweisungen auf der Lötlampe und der Gasflasche. Stellen Sie sicher, dass die Lötlampe ausgeschaltet ist, wenn Sie sie an die Gasflasche anschließen. Lesen und befolgen Sie alle Brandschutzvorkehrungen, die auf der Gasflasche angegeben sind. - Tragen Sie dicke Baumwollhandschuhe, um Verletzungen durch heiße Gegenstände zu vermeiden.
- Setzen Sie die Spitze des Metallstabs dem Feuer der Lötlampe aus, bis der Metallstab leuchtend rot ist.
- Decken Sie die betäubte Krabbe mit einem feuchten Baumwolltuch ab.
Anmerkungen: Decken Sie die Fühler der Krabbe ab, um unnötige Schäden zu vermeiden. - Halten Sie ein Auge der Krabbe mit einer sterilisierten Pinzette fest.
Anmerkungen: Sterilisieren Sie die Pinzette für den ersten Gebrauch in einem Autoklaven und desinfizieren Sie sie mit 70% Ethanol für die spätere Verwendung bei anderen Krabben. - Halten Sie die glühende flache Metallspitze auf das Auge der Krabbe und drücken Sie sie etwa 10−15 s lang leicht an, bis der Augenstiel eine orange oder rötlich-orange Farbe annimmt. Seien Sie vorsichtig, wenn Sie diesen Schritt durchführen, um Schäden an angrenzenden Strukturen zu vermeiden.
HINWEIS: Für die Durchführung der Augenstielablation nach der Kauterisationsmethode werden zwei Personen benötigt: eine, um die Krabbe zu halten, und eine andere, um die Ablation durchzuführen. - Desinfizieren Sie die Pinzette mit 70%igem Ethanolspray, um eine Kreuzkontamination zwischen Krabben zu vermeiden.
Anmerkungen: Führen Sie diesen Schritt nur mindestens 5 Minuten nach der Augenstielablation durch, um sicherzustellen, dass die Pinzette vor der Desinfektion mit 70%igem Ethanol abgekühlt ist, um potenzielle Brandgefahren zu vermeiden. - Nachdem Sie die Augenstielablation bei allen Krabben durchgeführt haben, tauchen Sie den heißen Nickelstahl-Metallstab (Schraubendreher) in Leitungswasser.
- Desinfizieren Sie das Handtuch vor der Wiederverwendung. Mehrere Handtücher können verwendet werden, um Zeit zu sparen.
Anmerkungen: Waschen Sie das Handtuch mit Leitungswasser und tauchen Sie es 5 Minuten lang in 30 ppm chloriertes Wasser. Waschen Sie das Handtuch dann erneut mit Leitungswasser und tauchen Sie es in eine 1 g/L Natriumthiosulfatlösung. - Bewahren Sie die Lötlampe nach dem Ausschalten an einem sicheren Ort auf und warten Sie, bis sie wieder Umgebungstemperatur erreicht hat (ca. 30 Minuten), bevor Sie sie trennen.
- Operationstechnik
- Führen Sie den Eingriff in einem gut belüfteten Bereich durch.
- Sterilisieren Sie zwei chirurgische Scheren und Pinzetten in einem Autoklaven.
- Gießen Sie 50 ml 70%iges Ethanol in ein 100-ml-Glasbecherglas.
- Trage dicke Baumwollhandschuhe.
- Halten Sie die betäubte Krabbe fest und decken Sie sie mit einem feuchten Baumwolltuch ab.
- Halten Sie ein Auge der Krabbe mit einer sterilisierten Pinzette fest.
- Schneiden Sie den Augenstiel schnell mit einer sterilisierten chirurgischen Schere ab.
HINWEIS: Die Hämolymphe kann durch den verwundeten Teil der Krabbe verloren gehen. - Tauchen Sie die Schere und Pinzette nach jedem Gebrauch in 70%iges Ethanol und trocknen Sie sie vor der Wiederverwendung mit Seidenpapier.
6. Pflege nach der Anästhesie
- Bereiten Sie 20 ppt gefiltertes Meerwasser vor und bewahren Sie es in einem Tank mit kontinuierlicher Belüftung auf.
- Verbinden Sie ein flexibles Rohr mit dem Oberbehälter für den Schwerkraftwasserfluss.
- Legen Sie die Krabbe unmittelbar nach der Ablation des Augenstiels in den Korb und setzen Sie die Krabbe fließendem Meerwasser (Umgebungswassertemperatur: 28 °C) aus dem Überkopftank aus.
- Halten Sie das Meerwasser fließen und überwachen Sie die Krabbe, bis sie sich spontan bewegen kann, was auf eine Erholung von der Narkose hinweist.
Anmerkungen: Meerwasser kann in einem Bodentank aufbereitet werden, und für den Wasserfluss kann eine Tauchwasserpumpe verwendet werden. - Halten Sie die Krabben einzeln in 20 ppt Meerwasser mit Belüftung für 30 min in einem Aquarium zur weiteren Beobachtung.
HINWEIS: Die geborgenen Krabben werden im anschließenden Brutbestandskulturprozess einzeln kultiviert.
7. Beobachtung der Reifung der Eierstöcke
- Aufzucht von Bruttieren;
- Übertragen Sie die reifen Krabben in einzelne kreisförmige 32-Liter-Tanks.
- Füttern Sie weiterhin zweimal täglich (morgens 09:00 Uhr und abends 20:00 Uhr) mit gehacktem Meeresfisch (tiefgefroren bei −20 °C) und entfernen Sie nicht gefressenes Futter vor der morgendlichen Fütterung.
- Aufzucht des Brutbestandes einzeln für 30 Tage bei 20 ppt Salzgehalt.
- Entfernen Sie den Kot und tauschen Sie täglich 10 % des Meerwassers (20 ppt) aus.
- Dissektion
- Reinigen Sie eine Präparierschale, eine Schere und eine Pinzette mit 70 % Ethanol.
- Betäuben Sie die Weibchen einzeln mit der 2-PE-Immersionsanästhesiemethode.
- Wählen Sie nach dem Zufallsprinzip neureife Weibchen aus (nach der Häutung frühreifer Weibchen), die noch keine Augenstielablation durchlaufen haben, um ihre Gonadenstadien zu bestätigen.
- Opfern Sie alle Versuchsweibchen mit Augenstielablatation einzeln und identifizieren Sie die Reifungsstadien der Keimdrüsen. Zerstören Sie die Brustganglien der Krabbe mit einer scharfen, sterilen Ahle. Entfernen Sie zuerst den oberen Panzer und dann das Hepatopankreas, um den Eierstock sichtbar zu machen. Beobachten Sie die Farbe der Eierstöcke und identifizieren Sie das Stadium der Eierstockreifung (Abbildung 2).
- Identifizierung der Reifungsstadien der Eierstöcke
- Beobachte die Farbe der Eierstöcke mit bloßem Auge oder unter einem Stereomikroskop.
- Identifizieren Sie die Reifungsstadien der Eierstöcke anhand der Färbung30: Die unreife (Stadium-1) zeigt eine durchscheinende oder cremeweiße Farbe; Die frühe Reifung (Stadium-2) zeigt eine blass bis hellgelbliche Farbe; iii) die Vorreifung (Stadium-3) eine gelbe bis hellorange Farbe aufweist; und (iv) der voll ausgereifte (Stadium-4) zeigt eine dunkelorange bis rötliche Farbe.
Ergebnisse
Reifung der Keimdrüsen
Bei 100% der präparierten Frauen (n = 6) wurde vor der Augenstielablation cremeweißes Ovarialgewebe (unreife Eierstöcke, Stadium 1) gefunden (Abbildung 2). Die Gonadenreifungsrate der weiblichen Augenstielkrebse (n = 63; 31 Weibchen mit der Kauterisationstechnik und 32 Weibchen mit der Operationstechnik) war höher als bei den weiblichen Krabben, die nach 30 Tagen individueller Aufzucht keiner Augenstielablation unterzogen wurden (n = 31) (
Diskussion
Dieses Protokoll wurde für die Augenstielablation der Schlammkrabbe, Scylla spp., entwickelt und kann als effiziente Methode zur Induktion der Gonadenreifung eingesetzt werden. Dieses Protokoll kann leicht für die kommerzielle Ovarialreifung von Schlammkrabben repliziert werden und kann implementiert werden, um die Latenzzeit (Zeit von einem Laichen zum nächsten) in der Samenproduktion von Schlammkrabben zu verkürzen.
Die Augenstielablation von Krebstieren (z. B. Süßwassergarnel...
Offenlegungen
Keiner der Autoren hat Interessenkonflikte.
Danksagungen
Diese Studie wurde vom malaysischen Bildungsministerium im Rahmen des Programms Higher Institution Centre of Excellence (HICoE) in Malaysia unterstützt, das vom Institute of Tropical Aquaculture and Fisheries der Universiti Malaysia Terengganu akkreditiert ist (Vot No. 63933 & Vot No. 56048). Wir bedanken uns für die Unterstützung der Universiti Malaysia Terengganu und der Sayap Jaya Sdn. Bhd. durch den Private Partnership Research Grant (Vot.-Nr. 55377). Eine außerordentliche Stelle als Academic Fellow von der Universiti Sains Malaysia nach Khor Waiho und Hanafiah Fazhan wird ebenfalls anerkannt.
Materialien
| Name | Company | Catalog Number | Comments |
| Aeration tube | Ming Yu Three | N/A | aquarium and pet shop |
| Airstone | Ming Yu Three | N/A | aquarium and pet shop |
| Autoclave machine | HIRAYAMA MANUFACTURING CORPORATION | N/A | MADE IN JAPAN |
| Bleaching powder (Hi-Chlon 70%) | Nippon Soda Co.Ltd,Japan | N/A | N/A |
| Blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Circular tank (32L) | BEST PLASTIC INDUSTRY SDN. BHD. | N/A | N/A |
| Cotton hand gloves (thick) | MR D.I.Y. Group Berhad | N/A | N/A |
| Cotton towel | MR D.I.Y. Group Berhad | N/A | N/A |
| Digital thermometer | Hanna Instrument | HI9814 | Hanna Instruments GroLine Hydroponics Waterproof pH / EC / TDS / Temp. Portable Meter HI9814 |
| Digital Vernier Caliper | INSIZE Co., Ltd. | N/A | |
| Dissecting tray | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Dropper bottle/Plastic Pipettes Dropper | Shopee Malaysia | N/A | N/A |
| Ethanol 70% | Thermo Scientific Chemicals | 033361.M1 | Diluted to 70% using double distilled water |
| Fiberglass tank (1 ton) | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Fine sand | N/A | N/A | collected from Sea beach of Universiti Malaysia Terengganu |
| First Aid Kits | Watsons Malaysia | N/A | N/A |
| Flat head nickel steel metal rod (Screw driver) | MR D.I.Y. Group Berhad | N/A | N/A |
| Formaldehyde | Thermo Scientific Chemicals | 119690010 | |
| Gas cylinder (butane gas) for blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Gas lighter gun (long head) | MR D.I.Y. Group Berhad | N/A | N/A |
| Glass beaker (100 mL)) | Corning Life Sciences | 1000-100 | |
| Ice bag | Watsons Malaysia | N/A | N/A |
| Perforated plastic baskets | Eco-Shop Marketing Sdn. Bhd. | N/A | N/A |
| PVC pipe 15mm | Bina Plastic Industries Sdn Bhd (HQ) | N/A | N/A |
| Refractometer | ATAGO CO.,LTD. | ||
| Refrigerator | Sharp Corporation Japan | N/A | Chest Freezer SHARP 110L - SJC 118 |
| Scoop net | MR D.I.Y. Group Berhad | N/A | |
| Seawater | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Siphoning pipe | MR D.I.Y. Group Berhad | N/A | N/A |
| Spray bottle | Mr. DIY Sdn Bhd | N/A | N/A |
| Stainless surgical forceps | N/A | N/A | N/A |
| Stainless surgical scissors | N/A | N/A | N/A |
| Submersible water pump | AS | N/A | model: Astro 4000 |
| Tincture of iodine solution (Povidone Iodine) | Farmasi Fajr Sdn Bhd | N/A | N/A |
| Tissue paper | N/A | N/A | |
| Transparent plastic aquarium | Ming Yu Three | N/A | aquarium and pet shop |
| Waterproof table | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
Referenzen
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H., et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K., et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y., et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production. Aquaculture Reports. 18, 100432 (2020).
- Waiho, K., et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R., et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T., et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S., et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G., et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I., et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N., et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N., et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C., et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H. -. Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q., et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A., et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8, (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K., et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus. Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S. -. K., Lee, S. -. G., Lee, J. -. M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity. Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X., et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C., et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C., et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 130 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H., et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Erratum
Formal Correction: Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs
Posted by JoVE Editors on 5/26/2023. Citeable Link.
An erratum was issued for: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs. The Introduction, Protocol, Discussion and References were updated.
The forth sentence in the third paragraph of the Introduction has been updated from:
The eyestalk ablation protocol in this work minimizes stress by using fully sedated crabs and minimizes physical injury to personnel from crab bites.
to:
The eyestalk ablation protocol in this work minimizes stress by using fully anesthetized crabs and minimizes physical injury to personnel from crab bites.
The start of the Protocol has been updated from:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia. The sacrifice of the experimental samples was done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Sexually pre-mature female mud crabs (orange mud crab S. olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
to:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia and was approved by the Universiti Malaysia Terengganu's Research Ethics Committee (Animal ethics approval number: UMT/JKEPHMK/2023/96). The sacrifice of the experimental samples was done according to the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Sexually pre-mature female mud crabs (orange mud crab Scylla olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
Section 4 of the Protocol has been updated from:
4. Cold-shock anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for cold shock anesthesia.
- Prepare 2 L of 4 °C to 1 °C seawater (20 ppt) in a transparent plastic aquarium. Maintain the temperature using (−20 °C) ice bags for cold shock anesthesia.
NOTE: Check the temperature with a digital thermometer. - Immerse the crab in the 4 °C seawater until sedated (about 3−5 min).
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement. The legs and chelipeds joints will still show minor movements when touched with forceps.
to:
4. Anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for anesthesia.
- After 5 min of acclimatization period, add 2-phenoxyethanol (2-PE) at 2 mL/L into each aquarium and allow 15 min of anesthesia treatment.
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement.
Section 5 of the Protocol has been updated from:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized (sedated) crab with a wet cotton towel.
NOTE: Cover all the tentacles of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
- After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Prepare the tincture of iodine solution in a dropper bottle.
NOTE: Tincture of iodine (iodine tincture or weak iodine solution) is made up of 2%-7% elemental iodine and potassium iodide, or sodium iodide, dissolved in ethanol and water. - Wear thick cotton gloves.
- Hold the sedated crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
- Apply two to three drops of iodine tincture to the wounded part of the eyestalk immediately after cutting it off.
NOTE: Tincture of iodine is used for healing and to prevent infection.
to:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle and keep it away from any fire-related sources, such as blow torch and red hot screwdriver. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized crab with a wet cotton towel.
NOTE: Cover the antennae of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color. Be careful when conducting this step to avoid damage to adjacent structures.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
NOTE: Only perform this step at least waiting for 5 min after the eyestalk ablation procedure to ensure the forceps are cooled down before disinfection using 70% ethanol to prevent potential fire hazards. - After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Wear thick cotton gloves.
- Hold the anesthetized crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
Step 7.2.2 of the Protocol has been updated from:
Sedate the females individually with the cold shock anesthesia method.
to:
Anesthetize the females individually with the 2-PE immersion anesthesia method.
The Discussion has been updated from:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,40,41. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,42. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,42,43. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,44. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique requires an additional step of disinfection using iodine. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,45,46.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. The application of a tincture of iodine can prevent infection of the wounded part. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,47. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,48,49.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,50,51. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,52. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
to:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Anesthesia via immersion in 2-phenoxyethanol was used as it is comparable to the use of tricaine methanesulfonate (MS-222) in arthopods but cheaper and does not require the use of additional buffer40. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,41,42. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,43. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,43,44. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,45. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique takes time for the wound to heal and this would allow for chance of infection. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,46,47.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,48. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,49,50. In addition, future research on pain assessment following eyestalk ablation on mud crabs is recommended to highlight the change in behaviours associated with pain and stress, as evident in freshwater prawn Macrobrachium americanum51.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,52,53. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,53. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
The References have been updated from:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
to:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Nachdrucke und Genehmigungen
Genehmigung beantragen, um den Text oder die Abbildungen dieses JoVE-Artikels zu verwenden
Genehmigung beantragenThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten