Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Methylene Blue Dye Injection in Mouse Embryonic Urinary Tract: A Method To Assess the Congenital Obstruction in the Urinary Tract
Dans cet article
Overview
In this video, we administer methylene blue dye injection into the urinary tract of a perinatal embryonic mouse to assess congenital anomalies related to urinary tract obstruction.
Protocole
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board
1. Preparation of Methylene Blue Dye Solution
- Measure 0.1 g methylene blue powder.
- Dissolve the methylene blue in 10 mL normal saline or 1x phosphate-buffered saline (PBS) completely by vortexing.
- Filter the 10 mg/mL methylene blue solution with a syringe filter (membrane pore size 0.45µm) to eliminate clogging during injection.
- Assemble a sterile scalp vein set (27 G x 3/4″) with a 3 mL disposable syringe and fill the syringe with 3 mL filtered methylene blue solution.
NOTE: To prevent hydrostatic pressure and subsequent dye flow, place the needle tip above the syringe filled with methylene blue solution.
2. Dissection of Prenatal Embryos
- Clean dissecting scissors and forceps with 70% ethanol.
- To collect perinatal embryos at E18.5 or E19.5, euthanize pregnant mice first using CO2 inhalation and then perform cervical dislocation according to NIH guidelines.
NOTE: The pregnant female mouse usually gives birth starting at E18.5, however, larger kidneys are easier to manipulate. Therefore, kidneys at E19.5 closer to birth are easier to perform this analysis on. However, pups with bilateral urinary tract obstructions die after birth. Depending on the characteristics of experimental mice, an appropriate collection day/time should be empirically determined. - Spray 70% ethanol on the ventral abdominal surface and then open the abdominal cavity ventrally using dissecting scissors and forceps.
- Lift the entire uterus and separate it from the body by cutting with dissecting scissors at the tips of the uterine horns.
- Rinse the entire uterus with 1x PBS in a Petri dish.
- Cut the uterus segmentally with dissecting scissors and remove placental decidua with dissecting forceps to expose the embryos in yolk sacs.
- Remove the yolk sac first and then remove the amniotic membrane with dissecting forceps to liberate the perinatal embryos.
- Decapitate an embryo with dissecting scissors. Wipe off excess blood with sterile gauze pads. Pin the embryo down with its ventral surface up on a dissecting microscope equipped with a digital camera for imaging. Collect its tail for genotyping if necessary.
NOTE: Perform injections one embryo at a time. - Carefully open the abdominal cavity of an embryo with forceps by tearing the skin. Then, carefully remove excess organs and tissues such as liver, stomach, and intestine with forceps by cutting them or pulling them out to expose the kidneys, the ureters, and the bladder that are located dorsally (Figure 1A). Absorb excess blood from the dissected embryo with sterile gauze pads if necessary.
NOTE: Excess blood interferes with identification of the renal pelvis for the dye injection.
3. Injection of Methylene Blue Dye into the Renal Pelvis and Monitoring Dye Flow
- Remove bubbles in the needle and tubing by expelling methylene blue solution from the needle tip by hydrostatic pressure. Lift the syringe containing the dye solution above the level of the needle tip to start flow, and then lower the syringe to stop flow.
- Insert the needle into the renal pelvis near the proximal ureter, taking care not to disturb it once placed. Perform dye injection into a kidney to determine its urinary tract obstruction.
NOTE: Perform dye injection into any abnormal kidney, first, by following the same way to determine its urinary tract obstruction. - Lift the syringe up about 20 cm to provide hydrostatic pressure and let 15 µL–60 µL of the dye solution flow. The flow rate will be about 3 µL/s if the syringe is raised to this height above the embryo.
- Monitor the blue color of the dye starting first in the renal pelvis, then in the length of the ureter, and finally in the bladder lumen.
NOTE: It takes about 5 s to see a weak dye color emerge within the bladder lumen if the ureter is properly inserted into the bladder. Allow the dye to accumulate at the blocked site about 15 s if no dye color appears in the bladder lumen. - Place the syringe down to stop dye flow and remove the needle from the kidney.
- Take images of the kidney, the ureter, and the bladder traced with dye solution using the camera and imaging program connected to a stereomicroscope.
- Make a record of hydronephrosis of the kidney, hydroureter, and the final position of the dye solution in a lab notebook.
- Perform injection of the contralateral kidney as described in step 3.1 to 3.5 and allow dye flow about 20 s in total.
NOTE: The bladder lumen will be filled with dye solution, indicated by a strong blue color, if the ureter is properly inserted into the bladder lumen. After assessment of urinary tract junction obstruction, the ureters and the bladder can be fixed for sectioning.
Résultats
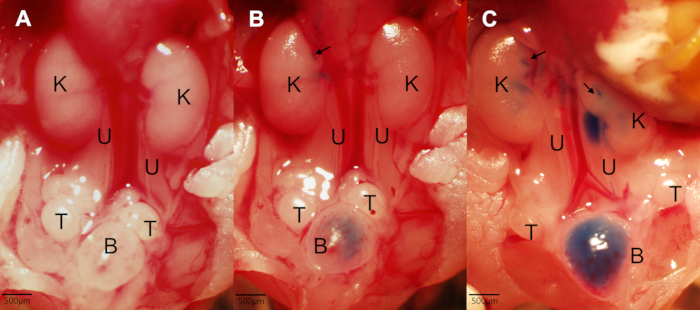
Figure 1. Examples of methylene blue dye injection into a normal urinary system. (A) A typical image of the urinary system from a perinatal embryo (E19.5) after removing excess tissue. Two kidneys, two ureters, and a bladder are seen with reproductive organs, here testes (B) An example of dye injection into a normal kidney. An injection point into t...
Déclarations de divulgation
matériels
| Name | Company | Catalog Number | Comments |
| Methylene blue | Sigma-Aldrich | M9140 | |
| Quality Biological Inc. NORMAL SALINE - 500ML | Fisherscientific | 50-983-204 | |
| 3 mL disposable syringe with BD Luer-Lok tip | BD | 309657 | |
| Acrodisc Syringe Filters with Supor Membrane | Pall corporation | 4614 | |
| Exel Scalp Vein (Butterfly) Sets; 27G x 3/4" 12" | EXELINT | 26709 | |
| Delicate Operating Scissors | Roboz Surgical Instrument Co. | RS-6702 | |
| Micro Dissecting Forceps | Roboz Surgical Instrument Co. | RS-5135 | |
| Dumont Tweezers; Pattern #5 | Roboz Surgical Instrument Co. | RS-5045 | |
| BD PrecisionGlide Needles 30G x 1/2" | BD | 305106 |
This article has been published
Video Coming Soon
Source: Yun, K. Assessing Urinary Tract Junction Obstruction Defects by Methylene Blue Dye Injection. J. Vis. Exp. (2017)