Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Electrophysiological Recordings from Drosophila Sensilla in Response to Volatile Odorants
Dans cet article
Overview
In this video, a live Drosophila fly is mounted in a tube for electrophysiological studies. Electrodes are positioned at the base of the sensillum to record specific neuronal responses to pheromones with the fly's olfactory receptor neurons.
Protocole
1. Preparation of the Hardware for at4 Recording
- Use a pipette puller instrument to prepare electrodes with aluminosilicate glass capillaries (O.D 1.0 mm, I.D. 0.64 mm). Blunt the tip of the reference electrode slightly with a pair of fine forceps to facilitate insertion into the clypeus of the fly (i.e. a rounded plate at the front of the fly head, above the mouthparts).
NOTE: 7-day-old WT(Wild type) males (Berlin) were used in this study. Use AHL saline solution as the electrolyte for both electrodes. - Prepare 1 L of AHL(Adult Hemolymph-Like) by mixing 900 mL of distilled water with 6.312 g of NaCl, 0.373 g of KCl, 0.337 g of NaHCO3, 0.1120 g of NaH2PO4, 1.892 g of Trehalose ּ2H2O, 3.423 g of sucrose, 1.192 g of HEPES(N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), and 8.2 mL of 1M MgCl2. Using distilled water, bring the total volume up to 1 L. Bring the pH to 7.4 using 1 N NaOH and sterilize the solution with a vacuum-driven filter system. For long-term storage, keep the AHL aliquots at 4 °C.
NOTE: Consistent deliveries of palmitoleic acid are contingent upon uniformity between cartridges. Each cartridge must be assembled in a reproducible manner. - Using a razor blade, remove 0.9 cm from the tip of a 200 µL pipette tip to create the first cartridge section, measuring 4.1 cm; refer to the dimensions detailed in Figure 1A. Use another 200 µL pipette tip and remove 1.7 cm and 1.5 cm from the tip and base to create the second cartridge section, measuring 1.8 cm (Figure 1A). Use a ruler to ensure reproducibility.
- Use a ⅛" hole puncher to cut discs from filter paper.
- Use forceps to place a filter paper disc at the tip of the second cartridge section. Visually confirm an opening in the cartridge tip through which air can pass.
- Attach the first and second cartridge sections, as shown in Figure 1A. Angle the second cartridge section downward to facilitate square aiming at the prep (Figure 1B).
- Connect the cartridge with the odor-delivery tube mounted on a micromanipulator.
NOTE: This design allows the cartridge to be swiveled outward to facilitate exchange (Figure 1C). - Set the constant humidified airflow to 2 L/min in one mass controller and the odorant flow to 500 mL/min in another mass controller.
- Using the software (see the Table of Materials), program the procedure to administer a 500 ms odor puff.
2. Preparation of Palmitoleic Acid Odorant Solutions for Delivery
NOTE: Or47b ORNs respond to both cis- and trans-palmitoleic acid. As palmitoleic acid is unstable at RT, stocks are stored at -20 °C and used within a month upon opening. Ethanol is the solvent of choice for palmitoleic acid.
- Use a vortex mixer to thoroughly mix 10 µL of cis- or trans-palmitoleic acid stocks or dilutions with 90 µL of 100% ethanol for ten-fold serial dilutions in 1.7 mL microtubes. Prepare fresh palmitoleic acid dilutions daily before experiments and use within a day.
NOTE: A glass vial is recommended for odorants not soluble in ethanol to prepare odor dilutions with other types of organic solvents. - Using a P10 micropipette, apply 5 µL of cis-palmitoleic acid solutions of the desired dilutions to the filter paper in each corresponding cartridge.
NOTE: The highest dosage (10-1) contains 450 µg of the compound. For trans-palmitoleic acid solutions, apply 4.5 µL instead so that the highest dosage (10-1 ) contains 450 µg of the compound. - Place the palmitoleic acid cartridges in a vacuum desiccator for 1 h at RT and 7.59 mmHg of pressure to completely evaporate the solvent.
NOTE: The cartridges can be used for up to 4 h at RT.
3. Preparation of Drosophila for Ready Access to the at4 Sensilla for In Vivo Electrophysiological Recordings
NOTE: WT flies (Berlin) are reared in standard cornmeal medium at 25°C in a 12:12 light-dark cycle. Upon eclosion, flies are separated by sex into groups of ten, whereby they are group-housed until 7 d of age. Or47b ORNs (Olfactory Receptor Neurons) in both male and female flies respond to palmitoleic acid. For simplicity, only male flies are examined in the current study.
- Assemble a fly-prep slide: on a glass slide, place a glass coverslip (18 x 18 mm2) on a small amount of modeling clay, forming a ~3° angle with the glass slide. Place double-sided tape on the inner edge of the coverslip and the area of the slide immediately below. Replace with fresh tape for every day of recording (Figure 2A).
- Use a fly aspirator15 to collect the fly of interest in the tubing and then fit a 200-µL pipette tip over the end of the tubing. Simultaneously, flick the tube forward while blowing air into it to push the fly to the end of the pipette tip. Use a razor blade to cut just below the body of the fly and 2 heads' lengths above the fly.
- Tamp the bottom of the pipette tip with modeling clay, pushing the fly upward until both the antennae and the clypeus are exposed (Figure 2B). To avoid killing the fly, add only enough clay to expose the antennae and aristae, as this prevents the fly's abdomen from being crushed. Furthermore, add clay slowly and gently to prevent any sudden constriction. Confirm that the fly is alive by checking for antennal or proboscis movement.
- Use forceps to maneuver the pipette tip that houses the fly. Orient the head so that the clypeus faces the observer's right. Adjust the prep along the coverslip using fine forceps until the lateral side of the antenna lies against the taped coverslip surface (Figure 2B).
- Place a holding rod on the arista to secure the antenna to the double-sided tape to prevent movement (Figure 2B).
NOTE: The holding rod is pulled from a borosilicate glass capillary with a pipette puller and held in position with modeling clay (Figure 2A). - Place the prep on the rig's stage (Figure 2C). Using the microscope, confirm that the trichoid is visible along the distal-lateral edge of the third segment of the antenna.
NOTE: The sensilla should be silhouetted against the background, simplifying their identification and facilitating recording (Figure 3). In this preparation, the most accessible trichoid sensilla are of the at4 type. - As described previously, 2, 15, keep the prep under constant humidified airflow (2 L/min) delivered via a separate air delivery tube about 2 cm from the prep (Figure 4).
4. Recording of at4 Sensillum Activity from Or47b ORNs in the at4 Trichoids in Response to Palmitoleic Acid
- Insert the reference electrode into the clypeus (Figure 3A). With a swift and smooth motion, ensure that the electrode is inserted just below the surface, where it can contact the hemolymph under the cuticle to avoid tissue damage.
- Lower the recording electrode slowly until it enters the same view plane as the target sensillum (Figure 3B). Record under a 50X objective lens.
NOTE: The tough trichoidal cuticle necessitates inserting the recording electrode into the sensillar base, whose wider area provides a larger target that reduces the likelihood that the electrode is deflected away (Figure 3B, inset). - Before applying odor stimuli to a sensillum, observe the following selection criteria: any trichoid failing to meet these standards should be rejected, and another sensillum should be chosen instead.
- Observe a high signal-to-noise ratio (see Figure 3C for an example).
- Observe identifiable spikes from at4A and at4C neurons (Figure 3C).
NOTE: The at4B spike amplitude appears very similar to at4A10 and cannot be identified easily without odor stimulation. - Observe that the basal firing rate of the at4A neurons is around or under 20 Hz.
NOTE: This criterion is specific for at4A because the basal firing rate for the neuron is higher than that of the basiconic ORNs. A much higher basal firing indicates that the neurons may have been damaged during electrode insertion.
- Connect the cartridge to the odorant-delivery tube. Start with the solvent control and then the odorants, from low to high concentrations. Use the micromanipulator to maneuver the cartridge towards the prep while aiming the cartridge squarely at the head of the prep. Visually confirm that the cartridge is pointed directly at the antenna (Figure 4) from a few millimeters away.
NOTE: The goal is to orient the opening of the cartridge directly at the antenna and position it near the target tissue. - Ensure that the odorant cartridge is separated from the recording electrode on its right by 1 - 2 mm and from the fly prep slide below by approximately 1 mm.
NOTE: In the setup described here, the odorant cartridge is closely bordered by the recording electrode, the reference electrode, and the fly-prep slide (Figure 4).
NOTE: Consider the distance between the cartridge and the recording/reference electrodes. A distance of around 4 mm is recommended. Unintentional contact may terminate the signal and break the tip of the recording electrode, damaging the current neuron and complicating further recordings.
NOTE: Consider the distance separating the cartridge and the fly-prep slide. Touching the coverslip may also dislodge the recording electrode, disrupting the recording. - Press "Record" in the data acquisition software to begin the recording.
NOTE: For each 10 s recording, a single 500 ms odor pulse is delivered directly to the antenna, as described in step 1.9. - After the odorant is applied, carefully retract the cartridge before replacing it with a cartridge of the next-highest concentration. Continue until the entire dosage range is obtained.
NOTE: Only one Or47b ORN is recommended to be recorded from each fly to avoid any possible effects of adaptation. - Thoroughly rinse the recording electrode with distilled water after finishing recording for the day.
- Analyze and plot the data using commercially available offline analysis software.
Résultats
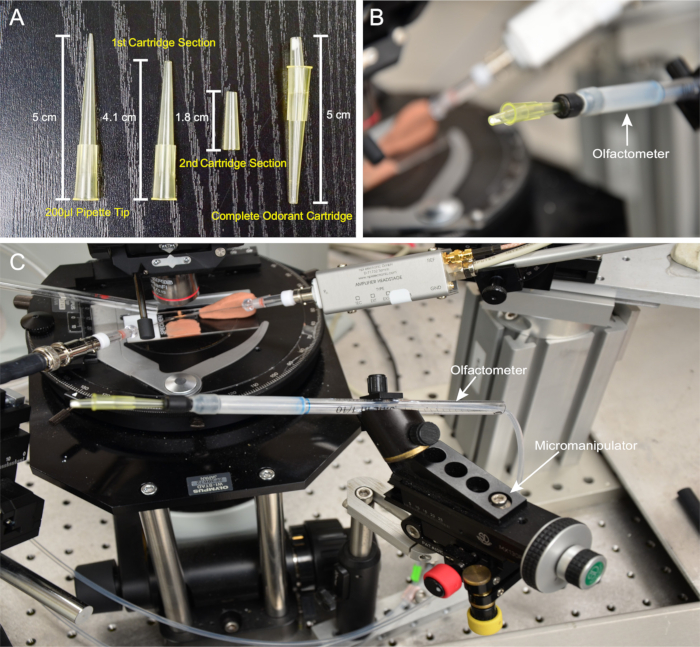
Figure 1: Cartridge and Olfactometer Setup. (A) Preparation of odor cartridges. From left to right: a standard 200-µL pipette tip, the first and second cartridge sections, and a completed odorant cartridge. (B) The cartridge connected to the olfactometer, showing the downward angling of the second section. (C) Olfactometer setup depicting the odor delivery tube mounted on the micromanipulator, with an attach...
Déclarations de divulgation
matériels
| Name | Company | Catalog Number | Comments |
| Prep Setup & Miscellaneous Materials | |||
| Pipette Puller Instrument | Sutter Instruments ,Novato CA USA | P97 | Pipette Puller |
| Borosilicate Glass Capillaries | World Precision Instruments, Sarasota FL USA | 1B100F-4 | to make holding rods |
| Aluminosilicate Glass Capillaries | Sutter Instruments, Novato CA USA | AF100-64-10 | to make electrodes |
| Superfrost Microscope Slides | Fisher Scientific, Pittsburgh PA USA | 12-550-143 | for fly-prep station |
| Permanent Double Sided Tape | Scotch St. Paul MN USA | NA | for fly-prep station |
| Upright microscope | Olympus, Shinjuku Tokyo Japan | BX51 | for recording rig |
| Plastalina modeling clay | Van Aken, North Charleston SC USA | B0019QZMQQ | for prep station and to stablize the holding rod |
| Rapid-Flow Sterile Disposable Filter Unit with SFCA Membrane, 0.45 mm | Nalgene, Rochester NY USA | #156-4045 | to sterilize AHL solution |
| 200 µL pipette tip | VWR Radnor, PA USA | 53508-810 | to make odor cartridges and fly prep |
| Filter Paper | Whatman, Maidstone Kent UK | 740-E | to make odor cartridges |
| Vacuum Desiccator | Cole-Parmer, Vernon Hills IL USA | VX-06514-30 | to vaporize ethanol solvent |
| cis-palmitoleic acid | Cayman Chemical, Ann Arbor MI USA | #10009871 (CAS # 373-49-9) | Or47b odorant |
| trans-palmitoleic acid | Cayman Chemical, Ann Arbor MI USA | #9001798 (CAS # 10030-73-6) | Or47b odorant |
| Ethanol | Spectrum Chemical MFG. New Brunswick NJ USA | E1028-500MLGL | to dilute palmitoleic acid |
| Odorant Cartridge Micromanipulator | Siskiyou, Grants Pass OR USA | MX130R | to position the olfactometer |
| Flow Vision software | Alicat, Tuscon AZ USA | FLOWVISIONSC | software to control flow rate |
| Mass Controller | Alicat, Tuscon AZ USA | MC-2SLPM-D | to control the flow rate for humidified air |
| Mass Controller | Alicat, Tuscon AZ USA | MC-500SCCM-D | to control the flow rate for odor stimulation |
| Clampex | Molecular Devices, Sunnyvale CA USA | Ver. 10.4 | Data acquisition software |
| Air delivery tube | Ace Glass, Vineland NJ USA | 8802-936 | to deliver humidified air |
| 50X objective lens | Olympus, Shinjuku Tokyo Japan | LMPLFL50X | recording rig |
| Clampfit 10 | Molecular Devices, Sunnyvale CA USA | Ver. 10.4 | software for spike analysis |
| Igor Pro 6 | WaveMetrics, Lake Oswego OR USA | Ver. 6.37 | software for data analysis |
| Audio Monitor | ALA Scientific Instruments, Farmingdale NY USA | NPIEXB-AUDIS-08B | Aurally reports individual spikes |
| Extracellular Amplifier | ALA Scientific Instruments, Farmingdale NY USA | NPIEXT-02F | to increase the amplitude of electrical signals |
| Valve Controller | Warner Instruments | VC-8 | to control the opening of the valve for odor stimulation |
| Recording Electrode Micromanipulator | Sutter Instruments, Novato CA USA | MP-285 | to position recording electrode |
| Headstage Amplifier | ALA Scientific Instruments, Farmingdale NY USA | EQ-16.0008 | to increase the amplitude of electrical signals |
| Oscilloscope | Tektronix, Beaverton OR USA | TDS2000C | Visual report of individual spikes |
This article has been published
Video Coming Soon
Source: Ng, R., et al. Electrophysiological recording from Drosophila Trichoid sensilla in response to odorants of low volatility. J. Vis. Exp. (2017).