Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Inducing Traumatic Injury in a Brain Slice Using a Shock Tube Device
Dans cet article
Overview
In this video, a shock tube device is used to generate a shockwave by rupturing a polymer diaphragm through controlled pressurization. The shockwave impacts brain slices contained in a secured bag, inducing traumatic injury to the brain slice.
Protocole
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Hippocampal Organotypic Slice Preparation and Culture
NOTE: This protocol allows the production of organotypic hippocampal slices. Ideally, no more than three animals should be euthanized and dissected in one session to ensure each step is done swiftly and to avoid compromising the quality of the slices. Use an aseptic technique throughout.
- Ensure the following steps are taken before starting the dissection protocol.
- Prepare and filter sterilize all solutions in advance using a 0.22 µm filter.
- Keep the solutions at 4 °C during the storage and throughout the brain dissection and hippocampal slice preparation by keeping the storage containers and Petri dishes on a metal heat sink sitting on wet ice at all times.
- Autoclave all metal instruments, rings, and paper tissues.
- Ensure all instruments and materials to be used for each step of the protocol are ready to use, laid out in advance, and sprayed with 70% ethanol. Then, allow them to cool down and dry.
- Euthanize a 5–7 day-old C57BL/6N mouse pup by cervical dislocation and briefly wipe the whole skin surface with sterile paper tissue soaked in 70% ethanol. Pat the skin dry and decapitate the pup using Mayo scissors.
- Cut the scalp skin with iris scissors along the midline of the head, starting in the occipital region and ending near the snout, and retract it laterally.
- Insert the tip of Vannas scissors in the foramen magnum and make two small lateral cuts on the bone along the transverse sinuses, then cut the skull along the midline up to the olfactory bulb and make two small cuts perpendicularly to the midline in this region.
- Using fine-point curved forceps, retract the flaps of bone laterally away from the midline. Carefully remove the brain with a small spatula and transfer it to a 90 mm silicone elastomer-coated Petri dish containing a "dissection medium."
NOTE: The dissection medium is Gey's balanced salt solution (5 mg/mL D-glucose, 1% antibiotic-antimycotic solution, 10,000 units/mL penicillin, 10 mg/mL streptomycin, and 25 µg/mL amphotericin B). - Remove the cerebellum and separate the cerebral hemispheres along the midline using a razor blade. Use a spatula to transfer the cerebral hemispheres to a new 90 mm silicone elastomer-coated Petri dish filled with fresh, ice-cold dissection medium. If more than one animal is to be used in one session, repeat Steps 1.2–1.6.
- Under a stereomicroscope, cut the olfactory bulb and the tip of the frontal cortex with a razor blade and separate the cerebral cortex from the rest of the cerebral tissue using fine-tipped forceps. This step leaves the hippocampus exposed on the medial surface of the cortical tissue. From this step onward, use a laminar flow tissue culture hood (ultraviolet sterilized and cleaned with a 70% ethanol solution).
- Apply ice-cold dissection medium to a flat plastic chopping disk and, using a spatula, position the brain tissue on the disk such that the medial surface of the cortex is facing up and the axis of the hippocampus is perpendicular to the axis of the chopping blade.
- Remove the dissection medium from the chopping disk as much as possible using a fine-tip Pasteur pipette.
- Cut the brain into 400 µm slices using a tissue chopper at a 50% chopping speed and force.
NOTE: This step must be completed as quickly as possible, as the brain tissue is not submerged in the dissection medium. - Replace the dissection medium in the silicone elastomer-coated Petri dish with the fresh ice-cold medium.
- Once the tissue chopper is finished, carefully submerge the brain tissue in a fresh dissection medium and transfer the cut tissue back to the 90 mm silicone elastomer-coated Petri dish using a straight-blade scalpel.
- Under a stereomicroscope, carefully separate the cortical slices using fine-tip forceps. For each slice, inspect the hippocampus for morphology and potential tissue damage resulting from the dissection or slicing.
- Separate the hippocampi from the entorhinal cortex and from the fimbria using fine-tip forceps and small Vannas scissors. Typically, around 6 to 8 hippocampal slices are generated per hemisphere.
- Transfer up to 6 hippocampal slices to a tissue culture insert using a cut Pasteur pipette and place it inside a 35 mm Petri dish. Ensure that the slices are spread a few millimeters apart (this will ensure each slice can be imaged individually).
- Immediately add ice-cold "growth medium" to the bottom of each Petri dish, under the tissue culture insert, just below the top of the tissue culture insert rim.
NOTE: Growth medium contains 50% Minimum essential medium Eagle, 25% Hanks' balanced salt solution, 25% horse serum, 5 mg/mL D-glucose, 2 mmol/L L-glutamine, 1% antibiotic-antimycotic solution, and 10 mmol/L HEPES(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), titrated to pH 7.2 with sodium hydroxide. - Change the growth medium the day after the dissection and every 2 to 3 days after that (use growth medium at 37 °C). Ensure that the growth medium is added sufficiently but not so much that it overflows over the tissue culture insert membrane, which could compromise the viability of the tissue slices.
- Before being used in experiments, keep the tissue slices in a humidified incubator at 37 °C with 5% carbon dioxide in the air for 12 to 14 days.
2. Preparation of the Hippocampal Organotypic Slices for the Experimental Blast TBI Protocol
NOTE: This section's steps, except imaging, take place in a laminar flow tissue culture hood.
- Insert the custom-made stainless-steel rings into a 6-well plate (one per well).
NOTES: The rings have a rim with a notch (which should sit in the 12 o'clock position) and fit snugly inside the wells, while the tissue culture inserts fit easily in this rim. Ensure that the rings are washed with a bactericidal disinfectant, thoroughly rinsed with purified water, autoclaved, and allowed to cool down in advance. - Fill the well of the 6-well plate with pre-warmed (37 °C) serum-free "experimental medium" with propidium iodide.
NOTE: Experimental medium with propidium iodide is 75% Minimum essential medium Eagle, 25% Hanks' balanced salt solution, 5 mg/mL D-glucose, 2 mmol/L L-glutamine, 1% antibiotic-antimycotic solution, 10 mmol/L HEPES and 4.5 µmol/L propidium iodide, titrated pH to 7.2 with sodium hydroxide. - Ensure that the medium level does not reach above the notch of the ring. Transfer the 6-well plate with the rings back to the incubator for 1 h to ensure that the medium is at 37 °C immediately before the tissue culture inserts are transferred.
- Transfer the tissue culture inserts with the organotypic slices from the 35 mm Petri dishes into the 6-well plate with the rings and the experimental medium with forceps.
- Make a dot on the insert rim in the 3 o'clock position with a permanent marker pen.
NOTE: The inserts will be removed from the 6-well plate during the experiment. This step facilitates returning the insert to its original position after the shock wave exposure and easily keeps track of each slice throughout the protocol. - Label each 6-well plate with a unique name & date and make a map of the wells of each plate, naming each well (e.g., A, B, C, etc.) and each slice in each well (e.g., 1, 2, 3, etc.), so that each slice has a unique identifier (e.g., A1, A2, A3, etc.).
- Transfer the 6-well plate to the incubator for 1 h immediately before imaging to ensure the slices are at 37 °C.
NOTE: Avoid overflowing medium or air bubbles underneath the tissue culture insert membrane, which could compromise the slices' viability. - 1 h after transferring to the experimental medium, image each slice individually using a fluorescence microscope (2X objective, NA 0.06) fitted with an appropriate excitation (BP 535/50 nm) and emission (LP 610 nm) filter to assess slice health before the injury protocol is carried out.
NOTES: The slices that exhibit areas of dense red staining at this stage should be considered to present compromised viability and should be excluded from further analysis (these typically represent less than 10% of the total number of slices generated). Ensure that imaging is performed sequentially and as swiftly as possible to minimize the time that the slices are outside the incubator (typically 6 wells should take just under 30 min to image). - Keep the lid of the 6-well plate on at all times. Some condensation may build up on the inside of the lid. If this happens, briefly use a hairdryer on the low setting.
- Ensure that all imaging conditions are identical on different days and between experiments.
NOTE: The aim of imaging is to quantify the tissue fluorescence; hence, this step is important to ensure the reproducibility of the results and allow the data to be compared.
3. Submersion and Transport of the Tissue Culture Inserts with the Hippocampal Organotypic Slices
- Immediately after imaging, in a laminar flow hood, one tissue culture is taken out of the 6-well plate using forceps.
- Carefully transfer the insert to a sterile polyethylene bag (3" x 5") pre-filled with 20 mL of warm (37 °C) experimental medium freshly bubbled with 95% oxygen and 5% carbon dioxide.
NOTE: Ensure that the oxygen and carbon dioxide enriched experimental medium was bubbled for at least 40 min with 95% oxygen and 5% carbon dioxide using a scintered-glass bubbler inside a Dreschel bottle and transferred into the sterile polyethylene bags inside a laminar flow tissue culture hood using a 20 mL syringe with a bacterial filter and a sterile filling tube (127 mm) attached. Seal the bags immediately and transfer them to a 37 °C incubator for at least 1 h before the tissue culture insert transfer. - Ensure that each sterile bag is correctly labeled (with the plate and well identification). Repeat this step for each tissue culture insert. Carefully exclude the air bubbles upon sealing the sterile bags (done securely by twisting the top of the bags and applying a plastic clamp).
- Return the sterile bags with the tissue culture inserts and the 6-well plates with experimental medium into the 37 °C incubator.
- After 1 h, carefully pack the sterile bags with the tissue culture inserts in plastic boxes inside a thermoregulated box filled with de-ionized water at 37 °C to keep the organotypic slices at physiologic temperature throughout the shock wave exposure protocol.
4. Preparation of the Shock Tube and Hippocampal Organotypic Slice Shock Wave Exposure
- Wear steel-toe protective boots, a laboratory coat, and gloves while preparing the shock tube and exposing the shock wave.
- Bolt the sterile bag holder frame to the shock tube distal flange, ensuring that the central hole is aligned with the shock tube outlet (using a blanking rod).
- Mount two pressure transducers radially: sensor 1 in the middle part of the driven section and sensor 2 in the distal flange of the shock tube (Figure 1A). Connect the pressure transducers to an oscilloscope through a current source power unit.
- Ensure that all shock tube release valves and flow controls are closed.
- Open the external compressed air line and charge the solenoid valve to 2.5 bar.
- Open the compressed air cylinder safety valve and slowly open the pressure regulator to increase the pressure to approximately 5 bar.
NOTE: The pressure set for this regulator should be slightly above the highest diaphragm bursting pressure. - Prepare the diaphragms by cutting 23 µm-thick polyester sheets into 10 x 10 cm2 squares. Prepare the handles using autoclave tape and stick them to the top and bottom of each diaphragm.
- Position one diaphragm (single slot of the double breech - single diaphragm configuration) or two diaphragms (both slots of the double breech - double diaphragm configuration) in the breech (Figure 1B).
- Center the diaphragms and clamp them using four M24 bolts and nuts, fastening them sequentially in a diagonally symmetric way and ensuring that the diaphragms are wrinkle-free.
- Clamp each sterile bag individually in a vertical position on the holder frame, ensuring that the surface of the tissue culture inserts with the organotypic hippocampal slices is facing the shock tube outlet and the tissue culture insert is centered inside the sterile bag (Figure 1C). Ensure the sterile bag is securely clamped all around to ensure firm and even immobilization.
- Wear ear defenders and safety spectacles when pressuring the shock tube. Switch on the current source power unit and oscilloscope to acquire the shock wave data (acquisition rate of 50 mega-samples/s, record length 20 ms, 1 million points) and close the solenoid valve.
- Using the flow control knob on the shock tube control panel, slowly pressurize the driver volume section of the shock tube for single diaphragm configuration or both the driver volume section and the double breech section of the shock tube for double diaphragm configuration.
NOTE: For single diaphragm configuration, the burst pressure will depend only on the diaphragm material and thickness, and the diaphragm will rupture spontaneously once the material bursting pressure is reached. For double diaphragm configuration, the bursting pressure will also depend on the gas pressure differential in both the driver and the double breech chambers, and for the diaphragms to burst in a controlled way, the double breech safety valve is opened manually once the target pressures are reached. - As soon as the diaphragm ruptures (producing a loud noise), quickly close the compressed air flow using the flow knob and open the solenoid valve.
NOTE: The total volume of the driver section can be modified by the insertion of blanking segments, allowing a wider range of shock wave peak overpressure and durations to be obtained. The ideal combination of shock wave parameters should be enough to cause tissue injury but not so high that it causes tissue culture to insert or sterile bag distortion or rupture. - Expose each sterile bag with tissue culture, insert it into a single shock tube wave, and return it immediately to the thermo-regulated box before a new sterile bag is taken from the box and clamped on the holder frame. Ensure that Steps 4.10–4.14 are performed as smoothly and swiftly as possible (within a few minutes) to prevent the experimental medium from cooling down, as temperatures below 37 °C may interfere with injury development.
- Once all tissue culture inserts have been exposed to a shock wave (or sham protocol), return them to the original 6-well plate and their respective well (inside a laminar flow tissue culture hood) and return to the incubator.
- Keep the 6-well plate in the incubator with 5% carbon dioxide in the air at 37 °C until further imaging.
- Include sham controls for each experiment and the slice shock wave exposure.
NOTE: Sham slices are treated identically to the slices exposed to a shock wave (sealed in the sterile bags with experimental medium, transported to the shock tube laboratory in the same thermo-regulated box, and suspended on the metal frame for an equivalent period of time), but the shock tube is not fired.
Résultats
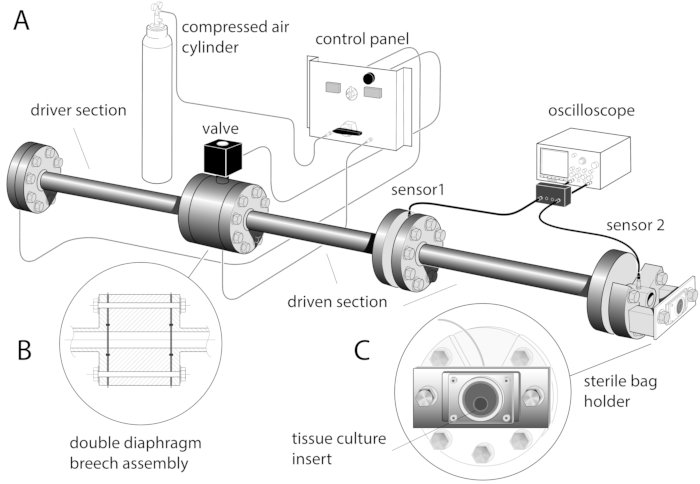
Figure 1: Schematic of the shock tube device with the sterile bag holder frame. (A). The shock tube is a 3.8 m long stainless-steel tube made of three 1.22 m long sections, connected by gaskets and flanges, with an internal diameter of 59 mm. (B) Inset shows the double breech assembly. One or two Mylar diaphragms can be clamped in the assembly with a seal provided by rubber o-rings. (C) Sterile b...
Déclarations de divulgation
matériels
| Name | Company | Catalog Number | Comments |
| Geys balanced salt solution | Sigma UK | G9779 | |
| D- glucose | Sigma UK | G8270 | |
| Antibiotic/antimycotic | Sigma UK | A5955 | |
| Minimum essential medium Eagle | Sigma UK | M4655 | |
| Hanks balanced salt solution | Sigma UK | H9269 | |
| Horse serum | Sigma UK | H1138 | |
| L-glutamine | Sigma UK | G7513 | |
| HEPES | VWR Prolabo, Belgium | 441476L | |
| Sodium hydroxide | Sigma UK | S-0945 | |
| Tissue culture inserts | Millicell CM 30 mm low height Millipore | PICM ORG 50 | |
| 6-well plates | NUNC, Denmark | 140675 | |
| Propidium iodide | Sigma UK | P4864 | |
| Sterile polyethylene bags - Twirl'em sterile sample bags | Fisherbrand | 01-002-30 | |
| Portex Avon Kwill Filling Tube 5" (127mm) | Smiths Medical Supplies | E910 | |
| Epifluorescence microscope | NIKON Eclipse 80i, UK | ||
| Microscope objective | Nikon Plan UW magn. 2x, NA 0.06, WC 7.5 mm | ||
| Microscope filter | Nikon G-2B (longpass emission) | ||
| Mylar electrical insulating film, 304 mm x 200 mm x 0.023 mm | RS Components UK | 785-0782 | |
| Pressure transducer | Dytran Instruments Inc. | 2300V1 | |
| Tissue chopper | Mickle Laboratory Engineering Co., Guildford, Surrey, United Kingdom. | Mcllwain tissue chopper | |
| Silicone elastomer | Dow Corning, USA | Sylgard 184 |
This article has been published
Video Coming Soon
Source: Campos-Pires, R., et al. A novel in vitro model of blast traumatic brain injury. J. Vis. Exp. (2018)