Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Method Article
L'extraction des lipides cellulaires pour ciblées dilution isotopique stable analyse de spectrométrie de masse chromatographie liquide-
Dans cet article
Résumé
This protocol will demonstrate the extraction and analysis of free and esterified bioactive fatty acids from cells. Fatty acids are accurately quantified using stable isotope dilution, chiral liquid chromatography, electron capture atmospheric chemical ionization multiple reaction monitoring mass spectrometry (SID-LC-ECAPCI-MRM/MS).
Résumé
The metabolism of fatty acids, such as arachidonic acid (AA) and linoleic acid (LA), results in the formation of oxidized bioactive lipids, including numerous stereoisomers1,2. These metabolites can be formed from free or esterified fatty acids. Many of these oxidized metabolites have biological activity and have been implicated in various diseases including cardiovascular and neurodegenerative diseases, asthma, and cancer3-7. Oxidized bioactive lipids can be formed enzymatically or by reactive oxygen species (ROS). Enzymes that metabolize fatty acids include cyclooxygenase (COX), lipoxygenase (LO), and cytochromes P450 (CYPs)1,8. Enzymatic metabolism results in enantioselective formation whereas ROS oxidation results in the racemic formation of products.
While this protocol focuses primarily on the analysis of AA- and some LA-derived bioactive metabolites; it could be easily applied to metabolites of other fatty acids. Bioactive lipids are extracted from cell lysate or media using liquid-liquid (l-l) extraction. At the beginning of the l-l extraction process, stable isotope internal standards are added to account for errors during sample preparation. Stable isotope dilution (SID) also accounts for any differences, such as ion suppression, that metabolites may experience during the mass spectrometry (MS) analysis9. After the extraction, derivatization with an electron capture (EC) reagent, pentafluorylbenzyl bromide (PFB) is employed to increase detection sensitivity10,11. Multiple reaction monitoring (MRM) is used to increase the selectivity of the MS analysis. Before MS analysis, lipids are separated using chiral normal phase high performance liquid chromatography (HPLC). The HPLC conditions are optimized to separate the enantiomers and various stereoisomers of the monitored lipids12. This specific LC-MS method monitors prostaglandins (PGs), isoprostanes (isoPs), hydroxyeicosatetraenoic acids (HETEs), hydroxyoctadecadienoic acids (HODEs), oxoeicosatetraenoic acids (oxoETEs) and oxooctadecadienoic acids (oxoODEs); however, the HPLC and MS parameters can be optimized to include any fatty acid metabolites13.
Most of the currently available bioanalytical methods do not take into account the separate quantification of enantiomers. This is extremely important when trying to deduce whether or not the metabolites were formed enzymatically or by ROS. Additionally, the ratios of the enantiomers may provide evidence for a specific enzymatic pathway of formation. The use of SID allows for accurate quantification of metabolites and accounts for any sample loss during preparation as well as the differences experienced during ionization. Using the PFB electron capture reagent increases the sensitivity of detection by two orders of magnitude over conventional APCI methods. Overall, this method, SID-LC-EC-atmospheric pressure chemical ionization APCI-MRM/MS, is one of the most sensitive, selective, and accurate methods of quantification for bioactive lipids.
Protocole
1. Standard and Internal Standard Mixes
- Before lipid extraction, one must prepare a standard mix (SM) and an internal standard mix (ISM). For the SM, aliquot the equivalent volume of 1 μg of each standard (25 total) listed in the reagent table below, using a calibrated syringe, into a 10 mL volumetric flask. Dry the standards under nitrogen and reconstitute in 10 mL of acetonitrile (ACN). The final concentration of the standard mix will be 100 pg/μL. Divide into 1 mL aliquots and store at -80°C until needed.
- From this standard mix, 6 standard dilutions can be made for a total of 7 calibration standards (1 pg/ μL, 2 pg/ μL, 5 pg/ μL, 10 pg/ μL, 20 pg/ μL, 50 pg/ μL, and 100 pg/ μL). These standards are to be run with each batch of samples prepared to develop the calibration curve used in quantitative analysis.
- For the ISM, aliquot the equivalent volume of 1 μg of each IS (14 total) listed in the reagent table below into a 10 mL volumetric flask, using a calibrated syringe, and dry down under nitrogen. Reconstitute the ISM in 10 mL of ACN and divide into 1 mL aliquots. The final concentration of the ISM should be 100 pg/μL. There is enough ISM to process 1000 samples.
- Store the SM and ISM at -80°C.
2. Collection of Media and Cell Lysate
- For each 10 cm plate of adherent cells (~ 1 X 107), collect 3 mL of media in a 10 mL glass centrifuge tube. Next, wash the cells 2 times with phosphate buffered saline (PBS). Add 1 mL of PBS to the plate and scrape the cells off the plate and pipet into a second 10 mL glass centrifuge tube. At this point cells and media may be stored if the procedure will not be continued at this time. Store samples at -80°C for long term storage or at -20°C for less than one week.
3. Extraction of Lipids from Media
- Free bioactive lipids are found in the cell media and these are easily extracted with a one-step l-l extraction procedure. To the collected media (2.1), add 10 μL of ISM. At this time, 8 additional 10 mL glass centrifuge tubes should be labeled #0-7 for the calibration standards. Add 3 mL of PBS to each of these tubes and then add 10 μL of the appropriate SM to tubes 1-7 (1-7 from 1.1).
- Allow the ISM to equilibrate in each calibration standard and sample for 10 minutes and then add 5 mL of diethyl ether. The samples and calibration standards are then placed in the shaker on low speed for 30 minutes at room temperature.
- After shaking, all calibration standards and samples are centrifuged at 1935 x g for 10 minutes. Next, using a Pasteur pipet, transfer the upper phase (organic) into a new 10 mL glass centrifuge tube and dry under nitrogen.
4. Extraction of Lipids from Cell Lysate
- Spin down the cell samples in the glass centrifuge tubes at 1935 X g for 5 minutes. Dispose of the supernatant; add 2 mL of PBS to each tube, and vortex briefly. Remove 25 μL of sample for protein quantification. Total protein will be used for cell lysate normalization.
- Next, transfer half of the lysate/PBS mixture into a separate 10 mL glass centrifuge tube. Add an additional 2 mL of PBS along with 10 μL of ISM to each sample. Follow the l-l extraction process described for media in 3.2 and 3.3. This aliquot will be used to measure free lipids in the cells and avoid alkaline degradation of lipids, such as the prostaglandins. This aliquot will not undergo steps 4.3 and 4.4 described below.
- The first step of the esterified lipid l-l extraction process requires the addition of 5 mL of CHCl3/MeOH (2:1) to each of the samples. Samples are set in a shaker on low speed for 30 minutes. After shaking, the samples are centrifuged at 1935 x g for 10 minutes. Next, using a Pasteur pipet, transfer the bottom (organic) layer of the l-l extraction into a new 10 mL glass centrifuge tube and dry the sample under nitrogen.
- Esterified lipids must be saponified before quantification. Once the samples are dry, add 0.5 mL of 0.4N KOH in 80% MeOH to each tube. The samples are then incubated at 60°C for 1 h. After the incubation, add 2 mL of PBS. Adjust the pH to 6 with concentrated HCl acid (~30 μL). Add 10 μL of ISM to each sample. The rest of the procedure is the same as lipid extraction from media and can be followed in 3.2 and 3.3.
- If these extractions are run simultaneously with the media samples the same calibration standards can be used to develop a calibration curve; however, if these samples are run at another time, calibration standards must also be processed as described in 3.1-3.3.
5. Pentafluorylbenzylbromide (PFB) Derivatization
- To the dried down samples and calibration standards, add 100 μL of anhydrous dichloromethane (CH2Cl2) followed by 100 μL of 20% (v/v) N,N-diisopropylethylamine in anhydrous CH2Cl2, and finally 100 μL of 20% (v/v) PFB in anhydrous CH2Cl2.
- After the addition of derivatization reagents, the samples are vortexed, incubated at 60°C for 1 h and dried under nitrogen. The samples and calibration standards may be stored at -80°C if they are not analyzed immediately or at -20°C for up to one week.
6. Stable Isotope Dilution Chiral Liquid Chromatography Electron Capture Atmospheric Pressure Chemical Ionization Mass Spectrometry (SID-LC-ECAPCI-MS) Analysis
- The dried down samples and calibration standards are reconstituted in 100 μL of hexane/ethanol (97:3). The entire sample can then be transferred to the HPLC vial with insert. Place the samples in the chilled autosampler (4°C) and create a sample list for analysis.
- Next, set up the HPLC and MS methods using the parameters described in Table 1 and Table 2 below. Table 3 contains specific compound information, including the MRM transition and collision energy (CE) for each lipid as well as the estimated retention time from the Waters 2695/Thermo Fisher TSQ Quantum Ultra analysis. The CE is determined by tuning each individual lipid during method development.
- A Chiralpak AD-H column (4.6 X 250 mm, 5 μ) heated to 30°C is used for the normal phase separation. The starting mobile phase is 98% hexane and 2% methanol/isopropanol (1:1). After the HPLC separation, methanol is added post-column (0.75 mL/min) to avoid deposition on the Corona needle that would cause a decrease in sensitivity. The MS is set in negative ion mode using the APCI source. If the source needs to be changed from ESI to APCI, be sure to insert a Corona needle.
- The first sample that should be run is always a hexane blank. This will equilibrate the column and also reveal if there is any contamination on the column that may interfere with the calibration standard and sample analysis.
- The next samples run should be the calibration standard samples in order of 0-7 (increasing SM concentration). These standards will be used to create a calibration curve containing the ratio of standard area/IS area on the y-axis and standard concentration or mass on the x-axis. From the y = mx + b equation derived from this plot, the ratio of sample area/IS area (y) can be used to determine sample (unknown) amount or concentration (x).
- The amount of bioactive lipids found in the media can be normalized to the total volume of media and the amount of lipids found in the cell lysate can be normalized to either total number of cells/plate or the amount of total protein/plate. Measures should be taken during 4.1 of the protocol to ensure that cells are counted or that protein is quantified.
7. Representative Results:
The chromatograms in Figure 1 are representative of a targeted lipidomics profile. The first panel (Figure 1A) shows 13- and 9-HODE along with the corresponding 13- and 9-oxoODE, derived from linoleic acid. In addition, 15-oxoETE, the oxidized metabolite of 15-HETE is also shown in the second panel. The bottom two panels show 20-HETE and the 20-HETE-IS, [2H6]-20-HETE. Figure 1B shows the separation and unique transitions determined for the HETE stereoisomers that result from enzymatic oxidation of AA or by ROS. Figure 1C is a representation of PGs, isoPs, and LTB4. Some of the standards do not have a deuterated IS commercially available; therefore, an IS that is similar in retention time is used for quantification. For instance, [2H8]-12(S)-HETE is used to quantify 11-HETE and 8-HETE species in addition to 12(R)-HETE and 12(S)-HETE.
From the representative chromatograms, it is evident that there is a small shift in the retention of the deuterated IS compared to the unlabeled standard. This shift to a later retention time is a result of the deuterium interaction with the normal phase column. Deuterium is more polar than hydrogen; therefore, there is a stronger interaction with the normal phase of the column. If this separation were taking place using a reversed phase column, the shift would be to an earlier retention time as a more polar moiety would have a weaker interaction with the reversed phase.
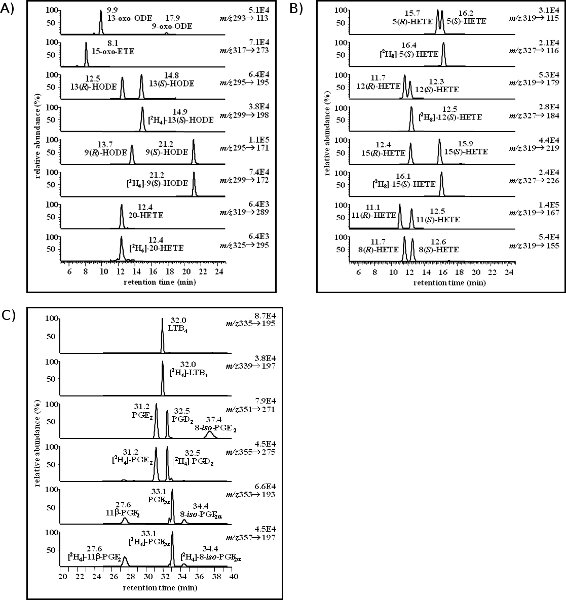
Figure 1. This figure is a representative example of a targeted lipidomics analysis. Figure 1A shows the total ion chromatograms (TIC) for several LA and AA oxidized metabolites including 13-oxoODE and 9-oxoODE in the first panel, 15-oxoETE in the second panel, 13-HODE and 9-HODE (panels 3 & 5) and 20-HETE (panel 7). The HODEs and 20-HETE have a corresponding deuterated IS in the panel below (4, 6, & 8). The TICs in Figure 2B show the separation and unique transitions of the HETE stereoisomers derived from AA. Figure 2C is a representation of PGs, isoPs, and LTs. The collision energy (CE), MRM transitions, and retention time (RT, min) were optimized using a Waters 2695 LC coupled to a Thermo Fisher TSQ Quantum Ultra and are listed in Table 3 for each compound.
| Time (min) | % A | % B |
| 0 | 98 | 2 |
| 3 | 98 | 2 |
| 11 | 96.4 | 3.6 |
| 15 | 92 | 8 |
| 27 | 92 | 8 |
| 30 | 50 | 50 |
| 38 | 50 | 50 |
| 39 | 98 | 2 |
| 45 | 98 | 2 |
Table 1. HPLC gradient conditions for the chiral normal phase lipidomics separation. Solvent A is 100% hexanes and Solvent B is methanol/isopropanol (1:1).
| Mass Spectrometry Parameter | Setting |
| Source | APCI |
| Mode | Negative Ion |
| Method Length | 40 min |
| MRM Scan Width | 0.002 m/z |
| MRM Scan Time | 0.15 sec |
| MRM Peak Width | 0.70 FWHM |
| Tube Lens | 164 V |
| Source CID | 10 eV |
| Q2 Collision Gas | 1.5 units |
| Vaporizer Temperature | 450°C |
| Capillary Temperature | 250°C |
| Discharge Current | 0 μA |
| Sheath Gas Pressure | 25 units |
| Ion Sweep Pressure | 3 units |
| Auxiliary Gas Pressure | 5 units |
Table 2. Listed above are the optimized mass spectrometry parameters for the measurement of bioactive lipids. These parameters are taken from a Thermo Fisher TSQ Quantum Ultra.
| Compound | Transition | CE (eV) | RT (min) |
| 13-oxoODE | 293.03 → 113.10 | 21 | 9.9 |
| 9-oxoODE | 293.03 → 113.10 | 21 | 17.9 |
| 15-oxoETE | 317.05 → 113.18 | 18 | 8.1 |
| 13(R)-HODE | 295.03 → 195.12 | 18 | 12.5 |
| 13(S)-HODE | 295.03 → 195.12 | 18 | 14.8 |
| [2H4]-13(S)-HODE | 299.03 → 198.12 | 18 | 14.9 |
| 9(R)-HODE | 295.03 → 171.10 | 18 | 13.7 |
| 9(S)-HODE | 295.03 → 171.10 | 18 | 21.2 |
| [2H4]-9(S)-HODE | 299.03 → 172.12 | 18 | 21.2 |
| 20-HETE | 319.03 → 289.10 | 18 | 12.4 |
| [2H6]-20-HETE | 325.03 → 295.10 | 18 | 12.4 |
| 5(R)-HETE | 319.04 → 115.04 | 16 | 15.7 |
| 5(S)-HETE | 319.04 → 115.04 | 16 | 16.2 |
| [2H8]-5(S)-HETE | 327.04 → 116.04 | 16 | 16.4 |
| 12(R)-HETE | 319.04 → 179.11 | 14 | 11.7 |
| 12(S)-HETE | 319.04 → 179.11 | 14 | 12.3 |
| [2H8]-12(S)-HETE | 327.13 → 184.11 | 14 | 12.5 |
| 15(R)-HETE | 319.04 → 219.12 | 13 | 12.4 |
| 15(S)-HETE | 319.04 → 219.12 | 13 | 15.9 |
| [2H8]-15(S)-HETE | 327.03 → 226.12 | 13 | 16.1 |
| 11(R)-HETE | 319.04 → 167.11 | 16 | 11.1 |
| 11(S)-HETE | 319.04 → 167.11 | 16 | 12.5 |
| 8(R)-HETE | 319.04 → 155.09 | 16 | 11.7 |
| 8(S)-HETE | 319.04 → 155.09 | 16 | 12.6 |
| LTB4 | 335.03 → 195.10 | 18 | 32.0 |
| [2H4]-LTB4 | 339.03 → 197.10 | 18 | 32.0 |
| PGE2 | 351.03 → 271.15 | 18 | 31.2 |
| [2H4]-PGE2 | 355.03 → 275.15 | 18 | 31.2 |
| PGD2 | 351.03 → 271.15 | 25 | 32.5 |
| [2H4]-PGD2 | 355.03 → 275.15 | 25 | 32.5 |
| 8-iso-PGE2 | 351.03 → 271.15 | 18 | 37.4 |
| 11β-PGF2 | 353.03 → 193.11 | 25 | 27.6 |
| [2H4]-11β-PGF2 | 357.03 → 197.11 | 25 | 27.6 |
| PGF2α | 353.03 → 193.11 | 25 | 33.1 |
| [2H4]-PGF2α | 357.03 → 197.11 | 25 | 33.1 |
| 8-iso-PGF2α | 353.03 → 193.11 | 25 | 34.4 |
| [2H4]-8-iso-PGF2α | 357.03 → 197.11 | 25 | 34.4 |
Table 3. This table lists the compounds detected in the total ion chromatograms of Figure 1A-C. The second column provides the transition used for MRM/MS, the third column lists the collision energy (CE, eV) and the last column lists the retention time (RT, min). Parameters were optimized using a Waters 2695 separation module coupled to a Thermo Fisher TSQ Quantum Ultra.
Discussion
The standards and internal standards used in this protocol provide a representation of a targeted lipidomics method. A Waters 2695 separation module and Thermo Fisher TSQ Quantum Ultra were used for the LC-MS analysis and the optimal parameter settings can be found in Tables 1 and 2. Additionally, this extraction protocol was designed for adherent cells, but can be modified for other cell types as well as other biological matrices including urine, blood, and tissue. Many lipid standards ...
Déclarations de divulgation
No conflicts of interest declared.
Remerciements
Most current bioanalytical methods available for the measurement of bioactive lipids are not as extensive as they do not include chiral normal phase chromatography or SID. Chiral normal phase LC is critical for the separation of enantiomers and for being able to distinguish between enzyme- or ROS-mediated metabolism. The use of SID ensures that human error or complications that arise during extraction or analysis are taken into account during quantification. These added components along with ECAPCI-MRM make this the most sensitive, selective method available for the analysis of bioactive lipids.
matériels
| Name | Company | Catalog Number | Comments |
| 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic-6,8,9,11,12,14,15-d7 acid | Cayman Chemical | 334250 | [2H7]-5-ox–TE Internal Standard |
| 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid | Cayman Chemical | 334230 | [2H8]-5(S)-HETE Internal Standard |
| 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid | Cayman Chemical | 334570 | [2H8]-12(S)-HETE Internal Standard |
| 15S-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid | Cayman Chemical | 334720 | [2H8]-15(S)-HETE Internal Standard |
| 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic-16,16,17,17,18,18-d6 acid | Cayman Chemical | 390030 | [2H6]-20-HETE Internal Standard |
| 9S-hydroxy-10E,12Z-octadecadienoic-9,10,12,13-d4 acid | Cayman Chemical | 338410 | [2H4]-9(S)-HODE Internal Standard |
| 13S-hydroxy-9Z,11E-octadecadienoic-9,10,12,13-d4 acid | Cayman Chemical | 338610 | [2H4]-13(S)-HODE Internal Standard |
| 9α,11α,15S-trihydroxy-prosta-5Z,13E-dien-1-oic-17,17,18,18,19,19,20,20,20-d4 acid | Cayman Chemical | 316010 | [2H4]-PGF2α Internal Standard |
| 9α,11α,15S-trihydroxy-(8β)-prosta-5Z,13E-dien-1-oic-3,3,4,4-d4 acid | Cayman Chemical | 316350 | [2H4]-8-iso-PGF2a Internal Standard |
| 9α,11β.,15S-trihydroxy-prosta-5Z,13E-dien-1-oic-3,3,4,4-d4 acid | Cayman Chemical | 10008989 | [2H4]-11β-PGF2 Internal Standard |
| 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic-17,17,18,18,19,19,20,20,20-d4 acid | Cayman Chemical | 312010 | [2H4]-PGD2 Internal Standard |
| 9-oxo-11α,15S-dihydroxy-prosta-5Z,13E-dien-1-oic-17,17,18,18,19,19,20,20,20-d4 acid | Cayman Chemical | 314010 | [2H4]-PGE2 Internal Standard |
| 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic-6,7,14,15-d4 acid | Cayman Chemical | 320110 | [2H4]-LTB4 Internal Standard |
| 9α,11,15S-trihydroxy-thromba-5Z,13E-dien-1-oic-3,3,4,4-d4 acid | Cayman Chemical | 319030 | [2H4]-TxB2 Internal Standard |
| 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid | Cayman Chemical | 34250 | 5-ox–TE Standard |
| 12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid | Cayman Chemical | 34580 | 12-ox–TE Standard |
| 15-oxo-5Z,8Z,11Z,13E-eicosatetraenoic acid | Cayman Chemical | 34730 | 15-ox–TE Standard |
| 5R-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid | Cayman Chemical | 34225 | 5(R)-HETE Standard |
| 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid | Cayman Chemical | 34230 | 5(S)-HETE Standard |
| 8R-hydroxy-5Z,9E,11Z,14Z-eicosatetraenoic acid | Cayman Chemical | 34350 | 8(R)-HETE Standard |
| 8S-hydroxy-5Z,9E,11Z,14Z-eicosatetraenoic acid | Cayman Chemical | 34360 | 8(S)-HETE Standard |
| 11R-hydroxy-5Z,8Z,12E,14Z-eicosatetraenoic acid | Cayman Chemical | 34505 | 11(R)-HETE Standard |
| 11S-hydroxy-5Z,8Z,12E,14Z-eicosatetraenoic acid | Cayman Chemical | 34510 | 11(S)-HETE Standard |
| 12R-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid | Cayman Chemical | 34560 | 12(R)-HETE Standard |
| 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid | Cayman Chemical | 34570 | 12(S)-HETE Standard |
| 15R-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid | Cayman Chemical | 34710 | 15(R)-HETE Standard |
| 15S-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid | Cayman Chemical | 34720 | 15(S)-HETE Standard |
| 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid | Cayman Chemical | 90030 | 20-HETE Standard |
| 9R-hydroxy-10E,12Z-octadecadienoic acid | Cayman Chemical | 38405 | 9(R)-HODE Standard |
| 9S-hydroxy-10E,12Z-octadecadienoic acid | Cayman Chemical | 38410 | 9(S)-HODE Standard |
| 13R-hydroxy-9Z,11E-octadecadienoic acid | Cayman Chemical | 38605 | 13(R)-HODE Standard |
| 13S-hydroxy-9Z,11E-octadecadienoic acid | Cayman Chemical | 38610 | 13(S)-HODE Standard |
| 9α,11α,15S-trihydroxy-prosta-5Z,13E-dien-1-oic acid | Cayman Chemical | 16010 | PGF2α Standard |
| 9α,11α,15S-trihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid | Cayman Chemical | 16350 | 8-iso-PGF2α Standard |
| 9α,11β,15S-trihydroxy-prosta-5Z,13E-dien-1-oic acid | Cayman Chemical | 16520 | 11β-PGF2 Standard |
| 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid | Cayman Chemical | 12010 | PGD2 Standard |
| 9-oxo-11α,15S-dihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid | Cayman Chemical | 14350 | 8-iso-PGE2 Standard |
| 9-oxo-11α,15S-dihydroxy-prosta-5Z,13E-dien-1-oic acid | Cayman Chemical | 14010 | PGE2 Standard |
| 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid | Cayman Chemical | 20110 | LTB4 Standard |
| 9α,11,15S-trihydroxythromba-5Z,13E-dien-1-oic acid | Cayman Chemical | 19030 | TxB2 Standard |
| Phosphate Buffered Saline | GIBCO, by Life Technologies | 14190 | |
| Diethyl Ether | Sigma-Aldrich | 346136 | |
| Dichloromethane | Acros Organics | 61030-1000 | anhydrous |
| N,N-diisopropylethyl amine | Sigma-Aldrich | 387649 | |
| Pentafluorylbenzyl bromide | Sigma-Aldrich | 101052 | |
| Hydrochloric Acid | Sigma-Aldrich | 320331 | |
| Potassium Hydroxide | Fluka | 00650 | |
| Acetonitrile | Fisher Scientific | A996-4 | |
| Methanol | Fisher Scientific | A454-4 | |
| Chloroform | Fisher Scientific | 366927 | |
| Hexane | Fisher Scientific | H303-4 | |
| Isopropanol | Fisher Scientific | A464-4 | |
| Ethanol | Decon Laboratories | 2716 | |
| Water | Fisher Scientific | W7-4 | |
| Pasteur Pipets | Fisher Scientific | 13-678-200 | |
| 10 mL Glass Centrifuge Tubes | Kimble Chase | 73785-10 | Screw cap |
| Phenolic Screw Caps | Kimble Chase | 73802-13415 | |
| Chiralcel ADH Column | Chiral Technologies | 19325 | |
| HPLC vials | Waters | 60000751CV | |
| HPLC inserts | Waters | WAT094171 |
Références
- Needleman, P., Turk, J., Jakschik, B. A., Morrison, A. R., Lefkowith, J. B. Arachidonic acid metabolism. Annu. Rev. Biochem. 55, 69-102 (1986).
- Nikolaev, V., Reddanna, P., Whelan, J., Hildenbrandt, G., Reddy, C. C. Stereochemical nature of the products of linoleic acid oxidation catalyzed by lipoxygenases from potato and soybean. Biochem. Biophys. Res. Commun. 170, 491-496 (1990).
- Panigrahy, D., Kaipainen, A., Greene, E. R., Huang, S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer. Metastasis. Rev. 29, 723-735 (2010).
- Basu, S. Bioactive eicosanoids: role of prostaglandin F(2alpha) and F-isoprostanes in inflammation and oxidative stress related pathology. Mol. Cells. 30, 383-391 (2010).
- Sanchez-Mejia, R. O., Mucke, L. Phospholipase A2 and arachidonic acid in Alzheimer's disease. Biochim. Biophys. Acta. 1801, 784-790 (2010).
- Hersberger, M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clin. Chem. Lab Med. 48, 1063-1073 (2010).
- Allayee, H., Roth, N., Hodis, H. N. Polyunsaturated fatty acids and cardiovascular disease: implications for nutrigenetics. J. Nutrigenet. Nutrigenomics. 2, 140-148 (2009).
- Capdevila, J. H., Falck, J. R. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. , 68-69 (2002).
- Ciccimaro, E., Blair, I. A. Stable-isotope dilution LC-MS for quantitative biomarker analysis. Bioanalysis. 2, 311-341 (2010).
- Blair, I. A. Electron-capture negative-ion chemical ionization mass-spectrometry of lipid mediators. Methods in Enzymology. 187, 13-23 (1990).
- Singh, G., Gutierrez, A., Xu, K., Blair, I. A. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl derivatives of biomolecules and drugs in the attomole range. Analytical Chemistry. 72, 3007-3013 (2000).
- Blair, I. A., Capriol, R. M., Gross, M. L. . Encyclopedia of Mass Spectrometry. , 283-307 (2005).
- Lee, S. H., Blair, I. A. Targeted chiral lipidomics analysis by liquid chromatography electron capture atmospheric pressure chemical ionization mass spectrometry (LC-ECAPCI/MS). Methods in Enzymology. 433, 159-174 (2007).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationExplorer plus d’articles
This article has been published
Video Coming Soon