Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Method Article
Modeling Alcohol Consumption in Rodents Using Two-Bottle Choice Home Cage Drinking and Microstructural Analysis
Dans cet article
Résumé
This protocol describes a standard intermittent-access two-bottle choice home cage drinking paradigm to model alcohol consumption in rats. In addition, it provides step-by-step instructions to augment the standard protocol with a DIY lick detection system that enables microstructural analysis of drinking behavior.
Résumé
Two-bottle choice home cage drinking is one of the most widely used paradigms to study ethanol consumption in rodents. In its simplest form, animals are provided with access to two drinking bottles, one of which contains regular tap water and the other ethanol, with daily intake measured by change in bottle weight over the 24 h period. Consequently, this approach requires no specialized laboratory equipment. While such ease of implementation is likely the greatest contributor to its widespread adoption by preclinical alcohol researchers, the resolution of drinking data acquired using this approach is limited by the number of times the researcher measures bottle weight (e.g., once daily). However, the desire to examine drinking patterns in the context of overall intake, pharmacological interventions, and neuronal manipulations has prompted the development of home cage lick detection systems that can acquire data at the level of individual licks. Although a number of these systems have been developed recently, the open-source system, LIQ HD (Lick Instance Quantifier Home cage Device), has garnered significant attention in the field for its affordability and user friendliness. Although exciting, this system was designed for use in mice. Here, we review appropriate procedures for standard and lickometer-equipped two-bottle choice home cage drinking. We also introduce methods for adapting the LIQ HD system to rats including hardware modifications to accommodate larger cage size and a redesigned 3D printed bottle holder compatible with standard off-the-shelf drinking bottles. Using this approach, researchers can examine daily drinking patterns in addition to levels of intake in many rats in parallel thereby increasing the resolution of acquired data with minimal investment in additional resources. These methods provide researchers with the flexibility to use either standard bottles or a lickometer-equipped apparatus to interrogate the neurobiological mechanisms underlying alcohol drinking depending on their precise experimental needs.
Introduction
Although moderate alcohol use has historically been associated with moderate health benefits, recent large-scale comprehensive studies have revealed that no amount of alcohol consumption is safe1,2. In fact, alcohol use is the 7th leading risk factor for death and disability globally1 with individuals who drink even small amounts of alcohol having increased risk for cancers, infectious diseases, and injury1,3. In the United States, deaths from alcohol use increased by almost 30% between 2016 and 20214. Importantly, the risk of death and disability increases monotonically with increased consumption1.
Although studies in humans have provided valuable insight into the neurobiology of alcohol use and misuse, the experimental control afforded by animal models is crucial for an in-depth understanding of the mechanisms underlying drinking behavior and risk for heavy drinking. These models are also valuable tools for the development of treatments aimed at reducing uncontrolled consumption. Two-bottle choice home cage drinking is one of the most widely used preclinical paradigms to study alcohol consumption in rodents. This is due, in large part, to its ease of implementation as it allows for the measurement of total voluntary alcohol drinking and preference without the need for specialized research equipment or complex analyses. Using this approach, researchers can collect measurements at their desired intervals (e.g., hours, days, weeks) by calculating the change in bottle weight from the beginning to the end of a drinking session. Variations on this basic method have been used by many in the field to facilitate low to moderate to binge levels of alcohol intake over both short and long periods of time. For example, continuous and intermittent two-bottle choice procedures have been used to facilitate low and moderate levels of alcohol intake, respectively, in both mice5 and rats6,7,8. The same paradigms can promote high levels of intake in alcohol-preferring strains7,9. Alternatively, an adaptation of this method that limits alcohol access to 2-4 h daily beginning approximately 3 h after the start of the dark cycle has been shown to engender binge drinking in mice (i.e., Drinking in the Dark)10,11. Additional variations on these methods have been used in conjunction with methods that facilitate alcohol dependence (i.e., chronic intermittent ethanol vapor exposure) to examine the escalation of voluntary alcohol drinking during withdrawal12,13,14,15,16,17,18,19.
Although widely adopted by the field, the standard approach of measuring alcohol intake by change in bottle weight is limited in resolution to total consumption per drinking session. Consequently, this approach is unable to capture drinking patterns, which are well known to impact both the risk for and severity of alcohol use disorder (AUD)20,21,22. Indeed, an individual's average intake over time can be a consequence of several heavy drinking episodes or many drinking episodes each associated with relatively low consumption and these nuances go undetected when consumption is measured as total intake per drinking session. Importantly, significantly more drinking episodes associated with large drinking bouts (i.e., gulping) are observed in individuals with AUD relative to healthy controls23 and data in nonhuman primates suggests that individuals who establish a pattern of alcohol consumption that includes large drinking bouts early in their drinking history are at significant risk for AUD22. Moreover, drinking episodes that include large bouts of consumption significantly increase risk for morbidity and mortality independent of AUD diagnosis21. Altogether, these data have led to increased interest in examining drinking patterns in addition to levels of overall intake in preclinical models and has prompted the development of a number of systems of varying complexity that are able to capture drinking data at the level of individual licks.
Among the first was the development of a two-bottle system equipped with photobeam lick detection designed for use in mice24. This system was subsequently adapted by a separate group for use in rats25. In the years since, variations on this theme have been used to develop similar systems contained within custom rodent housing26, that allow for lick detection of individual socially housed animals27,28, and that capture both feeding and drinking behavior29. Among these, LIQ HD (Lick Instance Quantifier Home cage Device)30 has garnered significant attention for offering a system for use in mice that parallels the ease of implementation and affordability of the system developed by Godnyuck et al.24. This system utilizes capacitive sensing to directly detect contact between the tongue and the conductive metal sipper tube, as opposed to indirect measures such as sipper approach or interaction that are afforded using the photobeam detection system. The authors provide data demonstrating that their capacitive sensing system affords significantly greater precision and sensitivity in lick detection, producing stronger correlations between lick number and change in bottle weight than photobeam detection. The authors further demonstrate the ability to use this system in conjunction with a continuous access two-bottle choice home cage drinking paradigm to capture measures of alcohol drinking microstructure in addition to measures of overall daily intake30.
Here, we describe procedures for two-bottle choice home cage alcohol drinking using a standard approach as well as methods that enable lick detection (Figure 1). We also introduce methods for building and implementing LIQ HDR - an adaptation of LIQ HD30 for use in rats that includes hardware modifications to accommodate larger cage sizes and a redesigned 3D-printed bottle holder compatible with standard off-the-shelf bottles. These methods provide researchers with the flexibility to use either a standard or lickometer-equipped approach depending on their experimental resources and data collection needs.
Below is a description of the materials needed and step-by-step instructions for capturing home cage drinking using the intermittent-access two-bottle choice drinking procedure adapted from Simms et al.7. In this procedure, rats are provided 24 h access to two drinking bottles 7 days a week. On Mondays, Wednesdays, and Fridays (MWF), rats receive one bottle containing water (H2O) and another bottle containing ethanol (EtOH). These bottles are removed on Tuesdays, Thursdays, and Saturdays (TRS) and replaced with two bottles of water. Rats drink from the same two bottles of water on Saturdays and Sundays. Note that these methods can easily be adjusted for alternate schedules of ethanol access (e.g., continuous, binge, etc.) if desired. Separate instructions are provided for standard and lickometer-equipped approaches.
Protocole
All representative data were collected using singly-housed adult Long-Evans rats with the approval of the University of Illinois Chicago Institutional Animal Care and Use Committee and in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Single housing is required in order to accurately monitor fluid consumption by each subject. Adult male (n = 38) and female (n = 22) Long-Evans rats were P50-80 upon arrival and allowed to acclimate to the vivarium for one week prior to starting the experiment. Rats had ad libitum access to standard chow (Teklad 7912, Envigo) and water unless otherwise stated.
1. Preparation before the experiment
- Label drinking bottles as H2O or EtOH. Then, label with a unique identifier for each rat one H2O and one EtOH bottle for MWF and two H2O bottles for TRS.
NOTE: Optionally, use different color tape to identify H2O and EtOH bottles to help avoid potential errors during bottle change. - Fill H2O bottles with drinking water 1 day prior to the experiment start date. Prepare EtOH solution by combining 190 proof EtOH with drinking water (20% v/v) 1 day prior to the experiment start date.
CAUTION: It is critical that the EtOH used is non-denatured, as denatured EtOH is poisonous. - Prepare an empty cage on the same shelving as the rats participating in the experiment. This cage will be used to calculate spillage.
- Equip cages with LIQ HDR system if using (see step 3, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, and Supplementary Figure 1).
- Determine the precise time of day for bottle change prior to the onset of the study. Bottles should be replaced at the same time every day in order to obtain an accurate measurement of 24 h intake.
NOTE: Previous research has shown that rodents exhibit the greatest degree of consumption early after the onset of the dark cycle10,31. Therefore, in order to capture intake during this highly active period (see step 2.3.1 for capturing binge-like drinking), it is recommended that bottle change occur 30-60 min after lights are turned off. Use red lamps during bottle change to allow for visualization without disruption of the light-dark cycle.
2. Daily procedures and maintenance
- On the first day of the experiment, do the following.
- Remove standard water bottles from cages.
- Optional: Rats often jostle the cage top as they are foraging for food, which could cause spillage that cannot be corrected by the empty spillage cage. The use of food cups inside the cage can alleviate this concern. If using, remove food from the cage top now, add a food cup inside the cage, and fill it with chow. Ensure that the floor space allotted to the rat still meets institutional guidelines based on the size of the rat cage and the size of the food cup.
- Indicate to care and husbandry staff, as applicable, that research personnel will provide food and water for the duration of the experiment.
- Cage changes can be disruptive to data collection. Perform cage changes in tandem with obtaining body weight measurements. Researchers choosing to do this should inform their care and husbandry staff accordingly.
- On subsequent days, follow the procedures outlined below.
NOTE: Remember that any movement of cages and bottles can create spillage. Therefore, it is important to be gentle with movements and use the same methods of applying and removing bottles on the spillage cage as are used for rat cages.- Enter the room prior to bottle change time, ensuring enough time to carry out the steps below, and place new bottles on cages at the appropriate session start time.
- Remove filter tops from cages. If MWF, proceed to step 2.2.3. If TRS, proceed to step 2.2.4.
- On Mondays, Wednesdays, and Fridays, remove TRS bottles from cage tops. Weigh MWF bottles (one EtOH and one H2O). Record the bottle's start weight for today's drinking session. Provide additional food to the cage top or food cups if necessary. At the designated session start time, place bottles on each rat's cage top with tape facing up so that rats do not chew the bottle label.
NOTE: Typically, water consumption is not recorded or reported for TRS, but researchers can optionally collect these data by obtaining on and off bottle weights as described below in step 2.2.3 and step 2.2.4 if desired. The side for the EtOH bottle should be alternated after each drinking session in order to avoid the development of potential side preference, which can inadvertently skew consumption data.- Optional: Previous work has shown that many rats exhibit binge-like drinking at the onset of the drinking session8,32,33. Most researchers capturing binge drinking during this time report intake during the first 30-60 min of the drinking session. To obtain intake data during this potential binge episode, follow the steps described below.
- Set a timer after bottles are applied to the last cage. Leave the room undisturbed during the timed period.
- Re-enter the room when the timer goes off. Remove EtOH and H2O bottles and record bottle weights. Return EtOH and H2O bottles to cages.
NOTE: This step is not required to capture binge episodes when using a system equipped for lick detection.
- On Tuesdays, Thursdays, and Saturdays, remove MWF bottles from cages at the designated session end time, corresponding to 24 h after the start time. Weigh each bottle and record the bottle end weight for the MWF drinking session. Obtain and record each rat's body weight. If necessary, provide additional food to the cage top or food cups. Place TRS H2O bottles on each rat's cage top with tape facing up so that rats do not chew the bottle label.
- Return filter tops to cages and leave the animal room.
NOTE: Optionally cover EtOH bottle sippers to prevent evaporation. If cage changes are being performed by research personnel, designate 1 day weekly during TRS duties to perform this task. Place each rat into a clean cage after obtaining body weight.
- Wash bottles and refresh fluids on a regular weekly or biweekly basis in accordance with the institution's animal care and husbandry procedures. Take care to perform these duties in a manner that does not interrupt drinking sessions. For example, TRS H2O bottles can be washed and refilled after removal on MWF. Similarly, MWF H2O and EtOH bottles can be washed and refilled after removal on TRS.
3. Lick detection during two-bottle choice experiments
NOTE: Researchers interested in capturing high resolution drinking data can modify the above procedure by adding lick detection capabilities to the bottles. LIQ HD30 is a low-cost do-it-yourself (DIY) system that allows for this type of data collection in mice. The procedures outlined below describe the modifications necessary to adapt LIQ HD for use in rats (referred to as LIQ HDR). See Table of Materials for a list of all commercially sold and custom 3D-printed parts necessary to construct one lick detection system, which can capture two-bottle choice data for up to 18 rats. The 3D print .stl files and instructions can be found at the author's website (www.ejgloverlab.com/3dprints).
- Setting up capacitive sensor units
NOTE: Each LIQ HDR system consists of three capacitive sensor units, which together capture lick data from 36 bottles in parallel corresponding to 18 single-housed rats. Each sensor unit consists of one differential I2C communication breakout board and one 12-key capacitive sensor board, which will connect to lickometers from six separate cages through six pairs of 2-pin cables color-coded for each lickometer (Figure 2A).- Gather the materials required for building all three capacitive sensor units, as shown in Figure 2B, along with required soldering tools.
- To start building sensor unit A, cover a 2-pin female connector cable with a 7 inch long, red heat-shrink tubing, insert its red and black wire ends to pin #0 and #1 on the sensor board (Figure 2C-1), and solder in place (Figure 2C-2). Make sure that the cable is at the front of the sensor board as shown in order to arrange the cages in numerical order from left to right in the vivarium without overlapping the cables and potentially disrupting recording (see step 3.6 and Figure 6).
- Cover five more cables with distinctly colored heat shrink tubing and solder to pin #2-11 as shown (Figure 2C-3). Make sure all red wires are soldered to even-numbered pins, which will connect to all bottles on the left side, and all black wires are soldered to odd-numbered pins, which will connect to all bottles on the right side.
- Cover the solder joints with hot glue on all sides for protection (Figure 2C-4). Label the connectors in numerical order, providing a unique ID for each lickometer (Figure 2C-4).
- To assemble sensor unit A, connect sensor board A to a communication breakout EndPoint with a 50 mm 4-pin cable, and connect the EndPoint to the 3.3 V output of the 5V-to-3V lever shifter with a 100 mm 4-pin cable (Figure 2D-1). Although both the sensor board and communication breakout board have two identical 4-pin connectors, it is still important to make sure that the wiring is exactly as shown (Figure 2D-1 and Figure 2F) in order to use the 3D printed sensor cases and the suggested vivarium setup (see step 3.6 and Figure 6). House sensor unit A inside the 3D printed case (Figure 2D-2). Gently close the top, taking care not to crush the level shifter cable (Figure 2D-3).
- Modify the I2C addresses of sensor boards B and C by soldering a jumper wire from the ADDR pin to the 3Vo or SDA pin as indicated (Figure 2E).
- Solder six color-coded female connector cables to sensor boards B and C using the same order used for board A. Label each connector with the corresponding lickometer ID number. Connect board B to the communication breakout MidPoint and C to an EndPoint with 50 mm 4-pin cables (Figure 2F).
- House sensor units B and C in their respective 3D-printed cases. Daisy-chain all three sensor units with Ethernet cables to complete the sensor setup (Figure 2G).
- Setting up the microcontroller interface
NOTE: The LIQ HDR microcontroller interface (Supplementary Figure 1A) is the same as described for LIQ HD30 with very minor modifications. The procedures outlined below provide a general overview of the steps necessary to set up the interface with modifications described in steps 3.2.6 and 3.2.8. Researchers are referred to the original protocol and the video tutorial provided on the author's GitHub page for more detailed step-by-step instructions30.- Gather the materials and soldering tools required to build the microcontroller interface (Supplementary Figure 1B). On the back side of the touch shield (Supplementary Figure 1C-1), create a solder jumper across the back lite solder pads (Supplementary Figure 1C-2).
- On the back side of the data logging shield, sever the jumper pad trace of the CS pin (Supplementary Figure 1D-1). On the front side of the data logger, solder a short jumper wire from the CS pin to pin #7 (Supplementary Figure 1D-2).
- To install the shield stacking headers, first stack them to the back of the touchscreen shield (Supplementary Figure 1E-1). Stack the data logger to the touchscreen, use tape to stabilize, and solder all the header pins to the data logger (Supplementary Figure 1E-2). Then remove the touchscreen.
- Stack the remaining 2 x 3 header to the microcontroller (Supplementary Figure 1F-1), then stack the data logger onto the microcontroller before soldering the header pins in place (Supplementary Figure 1F-2).
- Insert the coin battery into the data logger (Supplementary Figure 1G-1). Stack the touchscreen onto the data logger (Supplementary Figure 1G-1). Insert the microSD card along with its adapter into the data logger (Supplementary Figure 1G-2).
- To make the extra grounding wire, cut off one male connector from a jumper wire and strip the end to ~1/4 inch (Supplementary Figure 1H-1). Solder a strand of stripped solid-core wire to the jumper wire and cover the solder joint with heat shrink tubing (Supplementary Figure 1H-2).
- Plug the 4-pin jumper wires and extra grounding wire to the microcontroller (Black - GND, Red - 5V, Yellow - pin #21, Blue - pin #20, Supplementary Figure 1I-1). Secure the connections with hot glue (Supplementary Figure 1I-2). Optionally, wrap the jumper wires with heat shrink tubing (Supplementary Figure 1I-2).
- House the fully assembled microcontroller interface in the 3D-printed case, with the 4-pin jumper wires and grounding wire threaded through the opening at the top (Supplementary Figure 1J).
- Follow the original protocol for microcontroller interface software installation30.
- Building LIQ HDR lickometers
NOTE: The custom 3D print that holds the bottles is designed for use with a standard rodent drinking bottle with a sipper tube diameter of 8 mm (see Table of Materials, Figure 3A). This bottle holder can easily be adapted for use with similar bottles and sipper tubes with minor or no modifications.- Gather the materials, 3D prints, and soldering tools required for building one lickometer as shown in Figure 3B. Note that each LIQ HDR system accommodates a maximum of 18 lickometers for 36 bottles.
- To extend the male connector cable, strip both of its ends to ~1/4 inch in length, as well as the extension black and red wires (Figure 3C-1).
- Solder the extension wires to the connector cable (Figure 3C-2) and cover the solder joints with heat shrink tubing (Figure 3C-3). Cover the male connector cable with heat shrink tubing that is color-matched to a corresponding female connector cable on the sensor unit (Figure 3C-4).
- To be consistent with the sensor board wiring (Figure 2), thread the red and black wire through the left and right internal cable passage of the bottle holder, respectively (Figure 3D-1).
- Solder each wire to the very end of the 1 ¾ inch conducting copper tape (Figure 3D-2) and fold the copper tapes in half (Figure 3D-3).
- Unpeel the copper tape and use a pair of forceps to carefully maneuver it inside the sipper bracket (Figure 3E-1) before firmly adhering it to the inner walls of the sipper bracket (Figure 3E-2). Make sure that the solder side of the copper tape is adhered to the wall closer to the midline of the bottle holder, as indicated (Figure 3E-2). Push a sipper tube through the bracket to press the tape firmly against the wall and prime the bracket.
- To reinforce the solder side of the copper tape, which is not as adhesive and tends to peel off, apply a dollop of hot glue to the solder joint (Figure 3E-3). Smear the glue to cover both the copper tape and any adjacent exposed portion of the 3D print (Figure 3E-4).
- Thread the cable through the internal passage of the cable protector (Figure 3F). Be sure to select the appropriate cable protector (left- or right- facing) for each lickometer in order to use the suggested vivarium setup (see step 3.6 and Figure 6)."NOTE: Lickometers 5-6, 11-12, and 17-18, which are depicted in blue- or purple-colored cables in the current setup, require a left-facing cable protector (Figure 4D-1), whereas all other lickometers require a right-facing cable protector (Figure 4D-2).
- To install the cable clip, first fit one wire in the groove of one half of the cable clip (Figure 3G-1), then fit the other half over the second wire, and then tightly close both halves with the nut (Figure 3G-2).
- Label the male connector with the corresponding lickometer ID number (Figure 3H). Repeat these steps until all 18 lickometers are built.
- Installing LIQ HDR lickometers
NOTE: LIQ HDR is designed for installation on a standard shoebox rat cage with a metal wire top (Figure 4A-1).- Identify two pairs of rods with a center-to-center distance of ~75 mm on the wire cage top in order to accommodate the bottle holder. Use a pair of pliers to open slightly the metal rods around the sipper openings. Insulate these rods with electrical tape in order to prevent metal-to-metal contact between the sippers and the cage top (Figure 4A-2).
NOTE: Although the electrical tape is not accessible to rats and, therefore, not susceptible to chewing, we recommend discarding it at the end of the study and not reusing it between studies. - Install the lickometer and the 1/8-inch -thick laser acrylic panel to the cage top with two sets of M5 screws and nuts (Figure 4B). Make sure that the sipper openings on the lickometer and acrylic panel align, and that the sippers are not in contact with any part of the cage top.
- Secure the cable clip to the outer-most rod (~4 mm in diameter) of the wire cage top (Figure 4C). Adjust the cable clip if needed to make sure that the cage top rests snuggly on the cage bottom and that the cable is not being pulled or bent under too much tension. Make sure that the correct cable protector is being used for each lickometer (Figure 4D and see step 3.8.8).
- Adjust the sipper tubes as necessary so that only ~2 cm of the sipper extends from the cap (Figure 4E-1). To install bottles, insert the sipper tube through the sipper bracket of the bottle holder until it stops (Figure 4E-2). Only the very tip of the sipper tube should extend beyond the acrylic panel (Figure 4E-3). It is crucial that no other part of the sipper tube is exposed in order to avoid false positive licks from non-consummatory behaviors, such as the rat touching or playing with the sipper tube. A filter top should be able to rest snugly over the wire cage top and cable clip without disturbing the cable itself (Figure 4F).
NOTE: A thicker acrylic panel or a stack of multiple 1/8-inch panels can be used to fully enclose sipper tubes of longer lengths for experiments that require that sipper tubes extend further into the cage, such as when measuring drinking in young rats that may have trouble reaching a short sipper.
- Identify two pairs of rods with a center-to-center distance of ~75 mm on the wire cage top in order to accommodate the bottle holder. Use a pair of pliers to open slightly the metal rods around the sipper openings. Insulate these rods with electrical tape in order to prevent metal-to-metal contact between the sippers and the cage top (Figure 4A-2).
- Installing and using sipper blockers
NOTE: The sipper blocker (Figure 5A) is designed to restrict access by the rat to the sipper during bottle changes and to ensure accurate lickometer calibration at the onset of each drinking session.- Gather the 3D printed parts and an M5 screw, nut, and washer for one sipper blocker as shown in Figure 5B.
- Insert the SIPPER_Gear into the SIPPER_Base (Figure 5C-1). Make sure that the SIPPER_Gear is oriented exactly as shown in the 3D rendering inset. Insert the SIPPER_Worm into the opening at the top of the SIPPER_Base. Turn the worm clockwise while inserting until the head is flush with the base (Figure 5C-2).
- Screw the SIPPER_Nut to the end of the Worm from the bottom of the Base (Figure 5C-3). Use forceps to tighten the nut while turning the worm dial. Insert the round, narrow necks of the SIPPER_Blockers into the slots of the Gear (Figure 5C-4).
- Install the assembled sipper blocker onto the cage top using the M5 screw set (Figure 5D). Make sure that both sipper blockers align with the sipper openings.
- To block access to the sippers, turn the worm dial clockwise to lower the blockers until they completely cover the sipper openings (Figure 5E).
- To provide access to the sippers, turn the dial counter-clockwise until the blockers are in a vertical position perpendicular to the cage top (Figure 5F-1). Manually pull up on each blocker arm until the heads are just below the wire cage top. Rotate the blockers 90° so the heads are parallel to the metal rods on the cage top and the arms are locked in place (Figure 5F-2). Keep turning the dial counter-clockwise until both blockers are raised above the cage top (Figure 5F-3) and the sippers are fully accessible (Figure 5F-4).
- Using LIQ HDR system in the vivarium
NOTE: The following vivarium setup is optimized to capture two-bottle choice drinking using a single microcontroller interface connected to 18 standard shoebox rat cages. All electronic components, including the microcontroller interface, sensor units, and various cables, are secured to wall-mounted metal wire shelving that holds the cages (Figure 6A). Some modifications may be required to adapt to animal facilities and user needs.- Mount each sensor unit to the vivarium shelving using the custom 3D-printed mounting screw (Figure 6B). Make sure that each sensor unit is mounted directly underneath the cage with the yellow-colored lickometer in order to avoid unnecessary cable tension (Figure 6A).
- Arrange and secure each female connector cable making sure that they do not overlap with each other or bend excessively at their solder ends, which could result in poor lick detection (Figure 6B). If metal rod shelving is used, this can be easily achieved by cable tying the connector cables to separate rods on the shelf as depicted.
- Mount the microcontroller interface next to sensor unit A using the same 3D printed mounting screw and plug its 4-pin connector into the 5 V input port of the 5V-to-3V level shifter connected to sensor unit A (Figure 6C).
- Use electrical tape to secure the extra ground wire to a grounding source such as the metal shelving (Figure 6D).
- Arrange each cage based on the color and ID of the lickometer installed as per Figure 6A depiction in order to minimize cable overlap and tension.
- Securely connect all male lickometer connector cables to the matching female sensor connector cables (Figure 6E). Plug in the power supply to turn on the microcontroller interface (Figure 6F).
- On the main GUI (graphic user interface) page (Figure 6G), tap the Setting Symbol to open the Settings page. Users can change Lights ON and OFF time based on the vivarium schedule to ensure that the interface display automatically dims when the dark cycle begins (Figure 6H-1).
- Tap Edit Sensor Settings (Figure 6H-1) to adjust Touch Threshold to 8 and Release Threshold to 2, which we have found to enable the most consistent lick detection for rats using the current setup (Figure 6H-2). Note, however, that users may need to experiment with different settings if any modifications to the current system are introduced.
- Uncheck Auto Calibrate if data are only collected over 24 h period or less (Figure 6H-2).
4. Daily procedures for two-bottle choice home cage drinking using lick detection
- Follow the steps outlined in step 2 for standard two-bottle choice procedures with the following modifications.
- On Mondays, Wednesdays, and Fridays, before putting on the MWF bottles, use the sipper blockers to cover the sipper openings on all cages (see step 3.5.5 and Figure 5E).
- Before recording, select the correct Experimental Side on the main GUI page, which will be the side of the EtOH bottle for that day (Figure 6G).
- After installing all MWF bottles, tap START! on the main page (Figure 6G) to start calibrating sensors, during which users are recommended to take a step away from the system and make sure rats are not licking the sippers (Figure 6I-1). Calibration takes a few seconds to complete, after which the system enters the main recording page showing the cumulative lick numbers on each bottle for each lickometer (Figure 6I-2).
- Raise all sipper blockers above the cage top to allow access to the sippers (see step 3.5.6 and Figure 5F), and gently return all filter tops as described (see step 3.4.4 and Figure 4F).
- Tap Refresh to update the lick numbers on the screen, which will not update in real-time by itself (Figure 6I-2).
- On Tuesdays, Thursdays, and Saturdays, tap Save & Quit to stop recording and return to the main page (Figure 6I-2) before removing the MWF bottles.
- To remove SD card for data transferring, tap Eject SD before unplugging the SD card (Figure 6G). Insert the SD card into any computer and copy/paste the .csv data file named after the bottle ON date to that computer (Figure 6K). To remount the SD card for the next recording, insert the card into the interface and tap Mount SD (Figure 6J).
NOTE: We recommend backing up data files daily. - It is advisable to limit body weight measurements to once a week where compatible with the animal model and animal use protocol and perform this task on the day designated for cage change in order to minimize unnecessary disturbance to the lickometers and sensors. Unplug all male lickometer cables from the matching female sensor cables before moving cages. After weighing each rat, return the rat to a clean cage. Transfer the lickometer to a new cage top and reconnect each lickometer to the corresponding sensor when necessary.
- For experiments testing the effect of an intervention (e.g., drug injection) on EtOH intake and drinking microstructure, schedule such interventions prior to drinking session onset whenever possible. On occasions when this is not possible, researchers can interrupt lick detection by tapping Pause (Figure 6I-2) and resume data capture by tapping Resume after the intervention is complete. We suggest collecting bottle weights during each pause in the drinking session in order to capture changes in EtOH intake before and after the intervention as well as to account for spillage that may occur when removing the cage top to gain access to the animal.
- At the end of the study, all in-cage components should be disinfected thoroughly before being used with new animals to limit the transmission of contaminants between animals. We recommend gently spraying and wiping down the lickometer with 70% EtOH solution, while making sure not to disrupt the internal electrical components. The sipper blocker and acrylic panel can be washed with warm soap water.
5. Data analysis
- Prior to evaluating dependent measures, correct intake data for spillage that inevitably occurs as a result of changing bottles each day. To do so, average the change in bottle weight for EtOH and H2O bottles on the spillage cage over the course of the entire study period.
- Exclude any values greater than two standard deviations from the mean for each bottle. After excluding these outliers, subtract the calculated means from the changes in EtOH and H2O bottle weight obtained from bottles on cages containing animals. By subtracting this amount, researchers can be confident that the average loss resulting from spillage of a given solution is not considered in measures of consumption. Subtraction of the average spillage amount may result in a negative value for some animals on some occasions. Convert these to zero and interpret them as the absence of any intake for that solution.
- The primary dependent measures for two-bottle choice drinking experiments are EtOH intake (g/kg) and preference (%). Calculate intake from change in EtOH bottle weight (g) and normalize to body weight (kg) using the following steps.
- Convert change in bottle weight (g) to change in volume (mL) using the following equation :

The density of pure ethanol is 0.789 g/mL. Therefore, the contributing density of 20% EtOH is 0.1578 g/mL. H2O weighs 1 g/mL. Therefore, the contributing density of 80% H2O = 0.80 g/mL. Consequently, every mL of a 20% EtOH solution (v/v) is comprised of 0.1578 (EtOH) + 0.80 (H2O) = 0.9578 g/mL. Therefore, the final equation when using 20% (v/v) EtOH concentration is:

- Calculate the dose of ethanol consumed using the equation below. Use body weights obtained during the same week as the intake data in order to calculate ethanol consumed in terms of animal body weight.
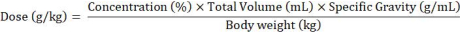
- Calculate preference using the following equation:
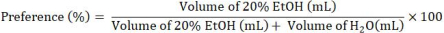
- Convert change in bottle weight (g) to change in volume (mL) using the following equation :
- Like its mouse counterpart30, LIQ HDR captures a variety of drinking microstructure variables, including lick duration, bout number, bout duration, bout lick number, and bout lick duration (Figure 6K). A drinking bout is defined as a series of licks that begins with at least 3 licks performed in less than 1 sec and ends after 3 sec have passed without additional licks. These measures can then be used to calculate the average individual lick duration, average individual bout duration, average number of licks per bout, average lick frequency, estimated inter-lick interval, and estimated inter-bout interval.
NOTE: The microcontroller interface provides these microstructure data at 1-min resolution in a .csv file (Figure 6K). Researchers can use the analysis app provided with the original LIQ HD protocol30 or their preferred post-processing platform to collate and summarize data according to research needs. Microstructure data are typically summated over the entire 24 h period to compare averaged differences on a macro level, or in 30 min to 1 h bin to examine drinking pattern variations across a light-dark cycle.
Résultats
Standard intermittent two-bottle choice home cage EtOH drinking data
Using the standard procedure (Figure 7A), researchers can capture 24 h intake (Figures 7B-D) as well as binge-like drinking if bottle weights are collected shortly after the onset of the drinking session (Figures 7E-F). By calculating ethanol intake relative to water intake, researchers can also obtain prefer...
Discussion
The current protocol provides step-by-step instructions for capturing ethanol drinking data using an intermittent-access two-bottle choice home cage procedure. This method can be implemented with relative ease and with little-to-no cost or need for specialized research equipment. Using the procedures provided here for constructing and implementing LIQ HDR - a lick detection system for use in rats - researchers can capture high-resolution drinking microstructure data in addition to standard measures of intake and preferen...
Déclarations de divulgation
All authors declare no conflicts of interest.
Remerciements
The authors thank Joseph Pitock and Katie Przybysz, PhD for technical assistance during LIQ HDR development. We also thank Nicholas Petersen and Marie Doyle, PhD for many helpful conversations during the development of LIQ HDR. This work was supported by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (P50 AA022538 and R01 AA029130 to EJG).
matériels
| Name | Company | Catalog Number | Comments |
| Commercially Available Components | Quantity | ||
| 12-Key capacitive touch sensor breakout (MPR121) | Adafruit | 1982 | 3 |
| 2-Pin cable matching pair (JST XH 2.5mm pitch) | Adafruit | 4872 | 18 |
| 4-Pin breadboard jumper (Qwiic) | SparkFun | PRT-17912 | 1 |
| 4-Pin cable (100mm Qwiic) | SparkFun | PRT-17259 | 1 |
| 4-Pin cable (50mm Qwiic) | SparkFun | PRT-17260 | 3 |
| 5V-to-3V level shifter breakout | Adafruit | 5637 | 1 |
| Capacitive touch shield (2.8inch) | Adafruit | 1947 | 1 |
| Copper conductive tape (1/4inch) | DigiKey | 4393-CFT-1/4-ND | 1 |
| Data logging shield | Adafruit | 1141 | 1 |
| Differential I2C communication breakout kit (QwiicBus) | SparkFun | KIT-17250 | 1 |
| Electrical tape | DigiKey | 3M156004-ND | 1 |
| Heat shrink tubing (3/16inch various colors) | Cable Ties and More | HS357-S10 | 6 |
| Heat shrink tubing kit (4inch black) | Jameco | 2095963 | 1 |
| Lithium coin battery (3V 12.5mm) | DigiKey | 1908-CR1220JAUCHSB-ND | 1 |
| M5 flat washer | Grainger | 54FN66 | 1 |
| M5 hex nut | Grainger | 6CA72 | 1 |
| M5 machine screw (12mm) | Grainger | 6GU88 | 1 |
| M5 machine Screw (25mm) | Grainger | 6GU91 | 2 |
| Male/male jumper wire | Adafruit | 1957 | 1 |
| Microcontroller board (Arduino Mega 2560 Rev3) | DigiKey | 1050-1018-ND | 1 |
| PETG filament (2.85mm silver) | MatterHackers | M-DDH-UZR2 | 2 |
| PLA filament (2.85mm black) | MatterHackers | M-SEW-RUAW | 2 |
| PLA filament (2.85mm silver) | MatterHackers | M-66P-6CLE | 1 |
| Power supply (DC 9V 1A) | Newegg | 9SIBPPKJYR5991 | 1 |
| SD/microSD memory card (8GB) | Adafruit | 1294 | 1 |
| Shield stacking headers | Adafruit | 85 | 1 |
| Solid-core wire (22AWG red) | Adafruit | 288 | 1 |
| Stranded-core wire (22AWG black) | Adafruit | 2976 | 1 |
| Stranded-core wire (22AWG red) | Adafruit | 3068 | 1 |
| Twist cap sipper tube with ball | Alternative Design | TCCN8.5-ST2.5SB | 38 |
| Water bottle (8oz) | Alternative Design | WB8FS | 38 |
| Custom 3D-Printed Components | Quantity | ||
| 3D-printed Arduino case bottom | N/A | ARDUINO_Bottom.stl | 1 |
| 3D-printed Arduino case top | N/A | ARDUINO_Top.stl | 1 |
| 3D-printed cable clip L | N/A | CLIP_L.stl | 18 |
| 3D-printed cable clip nut | N/A | CLIP_Nut.stl | 18 |
| 3D-printed cable clip R | N/A | CLIP_R.stl | 18 |
| 3D-printed lickometer bottle holder | N/A | LICKOMETER_Bottle Holder.stl | 19 |
| 3D-printed lickometer cable protector L | N/A | LICKOMETER_Cable Protector_L.stl | 6 |
| 3D-printed lickometer cable protector R | N/A | LICKOMETER_Cable Protector_R.stl | 12 |
| 3D-printed lickometer letters | N/A | LICKOMETER_Letters.stl | 19 |
| 3D-printed mount screw | N/A | Mount Screw.stl | 4 |
| 3D-printed sensor case A bottom | N/A | SENSOR_A_Bottom.stl | 1 |
| 3D-printed sensor case A top | N/A | SENSOR_A_Top.stl | 1 |
| 3D-printed sensor case B bottom | N/A | SENSOR_B_Bottom.stl | 1 |
| 3D-printed sensor case B top | N/A | SENSOR_B_Top.stl | 1 |
| 3D-printed sensor case C bottom | N/A | SENSOR_C_Bottom.stl | 1 |
| 3D-printed sensor case C top | N/A | SENSOR_B_Top.stl | 1 |
| 3D-printed sensor case letters | N/A | SENSOR_Letters.stl | 1 |
| 3D-printed sipper blocker | N/A | SIPPER_Blocker.stl | 36 |
| 3D-printed sipper blocker base | N/A | SIPPER_Base.stl | 18 |
| 3D-printed sipper blocker gear | N/A | SIPPER_Gear.stl | 18 |
| 3D-printed sipper blocker nut | N/A | SIPPER_Nut.stl | 18 |
| 3D-printed sipper blocker worm | N/A | SIPPER_Worm.stl | 18 |
| Laser-cut lickometer acrylic panel | N/A | LICKOMETER_Acrylic Panel_Sketch.dxf | 19 |
Références
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 392 (10152), 1015-1035 (2018).
- Fillmore, K. M., Stockwell, T., Chikritzhs, T., Bostrom, A., Kerr, W. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Annals Epidemiol. 17 (5 Suppl), S16-S23 (2007).
- GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 400 (10347), 185-235 (2020).
- Esser, M. B., Sherk, A., Liu, Y., Naimi, T. S. Deaths from excessive alcohol use - United States, 2016-2021. Morbidity Mortality Weekly Rep. 73, 154-161 (2024).
- Melendez, R. I. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 35 (4), 652-658 (2011).
- Wise, R. A. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 29 (3), 203-210 (1973).
- Simms, J. A., et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 32 (10), 1816-1823 (2008).
- Carnicella, S., Ron, D., Barak, S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 48 (3), 243-252 (2014).
- Loi, B., et al. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res. 34 (12), 2147-2154 (2010).
- Rhodes, J. S., Best, K., Belknap, J. K., Finn, D. A., Crabbe, J. C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behavior. 84 (1), 53-63 (2005).
- Thiele, T. E., Navarro, M. "Drinking in the dark" (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol. 48 (3), 235-241 (2014).
- Rimondini, R., Sommer, W., Heilig, M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Studies Alcohol. 64 (4), 445-449 (2003).
- Sommer, W. H., et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 63 (2), 139-145 (2008).
- Nentwig, T. B., et al. The lateral habenula is not required for ethanol dependence-induced escalation of drinking. Neuropsychopharmacol. 47 (12), 2123-2131 (2022).
- Becker, H. C., Lopez, M. F. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 28 (12), 1829-1838 (2004).
- Lopez, M. F., Becker, H. C. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 181 (4), 688-696 (2005).
- Griffin, W. C., Lopez, M. F., Becker, H. C. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 33 (11), 1893-1900 (2009).
- Hwa, L. S., et al. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 35 (11), 1938-1947 (2011).
- Vierkant, V., Xie, X., Wang, X., Wang, J. Experimental models of alcohol use disorder and their application for pathophysiological investigations. Curr Prot. 3 (6), e831 (2023).
- Greenfield, T. K., et al. Risks of alcohol use disorders related to drinking patterns in the U.S. general population. J Studies Alcohol Drugs. 75 (2), 319-327 (2014).
- Rehm, J., Greenfield, T. K., Rogers, J. D. Average volume of alcohol consumption, patterns of drinking, and all-cause mortality: results from the US National Alcohol Survey. Am J Epidemiol. 153 (1), 64-71 (2001).
- Grant, K. A., et al. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 32 (10), 1824-1838 (2008).
- Nathan, P. E., O'Brien, J. S. An experimental analysis of the behavior of alcoholics and nonalcoholics during prolonged experimental drinking: A necessary precursor of behavior therapy. Behav Ther. 2 (4), 455-476 (1971).
- Godynyuk, E., Bluitt, M. N., Tooley, J. R., Kravitz, A. V., Creed, M. C. An open-source, automated home-cage sipper device for monitoring liquid ingestive behavior in rodents. eNeuro. 6 (5), (2019).
- Frie, J. A., Khokhar, J. Y. An open source automated two-bottle choice test apparatus for rats. HardwareX. 5, e00061 (2019).
- Melo, M. C., Alves, P. E., Cecyn, M. N., Eduardo, P. M. C., Abrahao, K. P. Development of eight wireless automated cages system with two lick-o-meters each for rodents. eNeuro. 9 (4), (2022).
- Wong, K., et al. Socially integrated polysubstance (SIP) system: An open-source solution for continuous monitoring of polysubstance fluid intake in group housed mice. Addiction Neurosci. , (2023).
- Frie, J. A., Khokhar, J. Y. FARESHARE: An open-source apparatus for assessing drinking microstructure in socially housed rats. NPP-Digit Psychiatry Neurosci. 2 (1), 1-7 (2024).
- Zhao, Z., et al. INGEsT: An open-source behavioral setup for studying self-motivated ingestive behavior and learned operant behavior. bioRxiv. , (2024).
- Petersen, N., Adank, D. N., Raghavan, R., Winder, D. G., Doyle, M. A. LIQ HD (Lick Instance Quantifier Home Cage Device): An open-source tool for recording undisturbed two-bottle drinking behavior in a home cage environment. eNeuro. 10 (4), (2023).
- Trujillo, J. L., Roberts, A. J., Gorman, M. R. Circadian timing of ethanol exposure exerts enduring effects on subsequent ad libitum consumption in C57 mice. Alcohol Clin Exp Res. 33 (7), 1286-1293 (2009).
- Nentwig, T. B., Margaret Starr, E., Judson Chandler, L., Glover, E. J. Absence of compulsive drinking phenotype in adult male rats exposed to ethanol in a binge-like pattern during adolescence. Alcohol. 79, 93-103 (2019).
- Patwell, R., Yang, H., Pandey, S. C., Glover, E. J. An operant ethanol self-administration paradigm that discriminates between appetitive and consummatory behaviors reveals distinct behavioral phenotypes in commonly used rat strains. Neuropharmacology. 201, 108836 (2021).
- Bito-Onon, J. J., Simms, J. A., Chatterjee, S., Holgate, J., Bartlett, S. E. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 16 (3), 440-449 (2011).
- Mill, D. J., Bito-Onon, J. J., Simms, J. A., Li, R., Bartlett, S. E. Fischer rats consume 20% ethanol in a long-term intermittent-access two-bottle-choice paradigm. PloS One. 8 (11), e79824 (2013).
- Carnicella, S., Amamoto, R., Ron, D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 43 (1), 35-43 (2009).
- Moore, C. F., Lynch, W. J. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav. 132, 1-9 (2015).
- Pitock, J. R., et al. Presence of distinct operant phenotypes and transient withdrawal-induced escalation of operant ethanol intake in female rats. bioRxiv. , (2024).
- Carnicella, S., Yowell, Q. V., Ron, D. Regulation of operant oral ethanol self-administration: a dose-response curve study in rats. Alcohol Clin Exp Res. 35 (1), 116-125 (2011).
- De Oliveira Sergio, T., et al. Sex- and estrous-related response patterns for alcohol depend critically on the level of compulsion-like challenge. Prog Neuro-Psychopharmacol Biol Psychiatry. 133, 111008 (2024).
- Foo, J. C., Skorodumov, I., Spanagel, R., Meinhardt, M. W. Sex- and age-specific effects on the development of addiction and compulsive-like drinking in rats. Biol Sex Diff. 14 (1), 44 (2023).
- Moolten, M., Kornetsky, C. Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol. 7 (3), 221-225 (1990).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon