A subscription to JoVE is required to view this content. Sign in or start your free trial.
Concurrent Electroencephalographic and Local Field Potential Recordings in an Anesthetized Rat
In This Article
Overview
This video demonstrates the procedure for concurrent recordings of co-localized electroencephalography (EEG) and local field potentials (LFPs) in an anesthetized rat. The process involves preparing the rat and attaching stimulating electrodes to its whisker pads. An EEG spider electrode is placed on the rat's skull for surface EEG measurements. At the same time, a multi-channel microelectrode is inserted into a specific brain region through a burr hole in the skull to record neural activity from a localized brain area.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Co-localized EEG/LFP Setup
- Clean and dry the skull surrounding the burr hole using a cotton swab.
- Carefully place the conductive EEG paste on one flat side of an EEG spider electrode. Leave a small hole clear of the EEG paste on the spider electrode to allow a multi-laminar microelectrode to pass through the hole without contacting the paste and the spider electrode. This prevents electrical contact between the EEG electrode and the microelectrode.
- Align the spider electrode to the burr hole in the skull, with the EEG paste facing the skull.
- Carefully press the spider electrode onto the skull, making firm contact with the skull via the EEG paste. Remove any paste obscuring the burr hole using a needle on a syringe.
- Remove excessive EEG paste beyond the periphery of the spider electrode so that the contact between the spider electrode and the skull is spatially constrained to the size of the electrode (Figure 1B).
- Smear EEG paste onto the reference electrode for the EEG and place it securely inside the incision at the back of the rat’s neck.
- Connect the EEG electrodes to the preamplifier via a passive signal splitter for low impedance signals (Figure 2). Make sure the impedance of the spider electrode is below 5 kΩ. If it is not, check that the EEG paste is in good contact with the skull and the electrode is firmly pressed to the EEG paste. Add more EEG paste if necessary.
- Mount a micromanipulator arm on the stereotaxic frame. Connect a linear 16-channel microelectrode (100 µm spacing, area of each site 177 µm2) to a 16-channel acute headstage clipped securely onto the micromanipulator arm.
- Smear EEG paste onto the reference electrodes for the EEG and microelectrode, then securely place them inside the incision (Figure 1A).
- Adjust the angle of the micromanipulator arm so that the microelectrode is perpendicular to the cortical surface. This angle is normally between 25-35 °.
- Lower the microelectrode under a microscope by turning the micromanipulator knobs so that the tip of the microelectrode is aimed at the tiny opening at the bottom of the burr hole until the uppermost electrode just penetrates the cortical surface. Care must be taken to avoid forcing the microelectrode onto the surface of the dura as this would break the electrode.
- Couple the 16-channel microelectrode to a preamplifier connected to a data acquisition unit via a fiber optic cable (Figure 2).
- Turn on the preamplifier, the data acquisition unit, and the computer connected to the unit. Turn on the stimulator box.
- Insert the microelectrode normally to the cortical surface by slowly turning the z-axis knob of the micromanipulator to a depth of 1,500 µm.
- Micro-adjust the depth by applying a train of stimulus to the whisker pad and observing the 16-channel evoked LFP on a PC monitor using the software of the data acquisition unit installed on the PC. Carefully turn the z-axis knob on the micromanipulator until the highest amplitude of the evoked LFP occurs around channel 7 (as this coincides with layer IV in the cortex).
NOTE: Ipsi-lateral EEG electrode setup: For some experiments, a second spider electrode was placed on the ipsi-lateral side of the intact skull above the barrel cortex. This setup allowed bilateral EEG recording during the resting state to investigate the effect of the burr hole on the EEG signal.
NOTE: The surgical procedure to set up the EEG electrode is identical to that described above, except the temporalis muscle on each side of the head was carefully separated from the skull, sutured back and tied securely to the corresponding side of the stereotaxic frame.
NOTE: The concurrent EEG/LFP setup is also identical to that described above, with an additional step that a second spider electrode is loaded with the EEG paste, then pressed firmly to the skull above the ipsi-lateral barrel cortex.
תוצאות
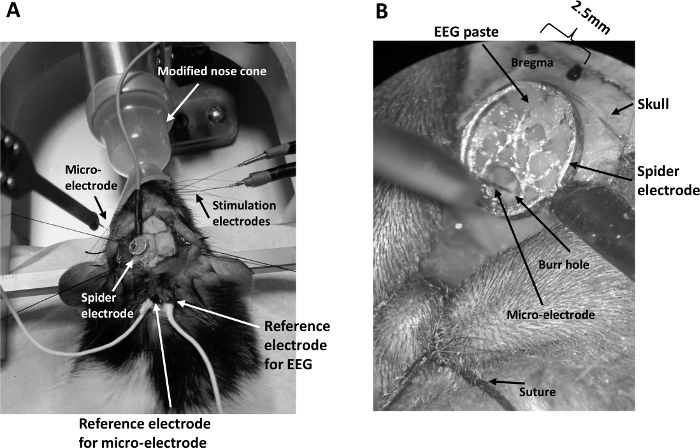
Figure 1: General setup for concurrent EEG/LFP recording. (A) The setup consists of a modified nose cone for ease of whisker pad stimulation under isoflurane anesthesia, two stimulating electrodes inserted into the whisker pad, a spider electrode positioned on the skull above the barrel cortex contra-lateral to the stimulating electrodes, a multi-channel microelectrode inserted i...
Disclosures
Materials
| Name | Company | Catalog Number | Comments |
| Female Lister Hood rats | Charles Rivers | ||
| Spider electrode | Unimed Electrode Supplies Ltd | SCS24-426 | |
| EEG paste: Ten20 | Unimed Electrode Supplies Ltd | 10-20-S | |
| Stereotaxic holder with dual micromanipulator arms: Dual Manipulator Stereotaxic Frame with 18° Ear Bars | WPI (World Precision Instruments) | 502603 | |
| Stainless steel stimulating electrodes | PlasticsOne | E363/1/SPC | |
| Isolated current stimulator | Made in House | ||
| 16-channel micro-electrode, 100 μm spacing, area of each site 177 μm2 | NeuroNexus | A1x16-10mm-100-177-A16 | |
| 16-channel acute headstage | Tucker David Technologies Inc., TDT | RA16AC-Z | |
| Pre-Amplifier: Z-Series 64-Channel Neuro-Digitizing Preamp | TDT | PZ5-64 | |
| Passive signal splitter: 32-Channel Splitter Box for PZ5 | TDT | S-BOX_PZ5 | |
| Data acquisition unit: RZ2 BioAmp Processor. Z-Series 4-DSP ultra high performance processor | TDT | RZ2-4 | |
| Isoflurane | National Vet Services Limited | 50878 | |
| Small animal isoflurane anaesthetic system | WPI | EZ-B800A | |
| Thermostatic heating pad: Rat Blanket System 230V | Harvard Apparatus UK | 50-7221-F | |
| Ophthalmic ointment: Optixcare eye lube | Viovet | 203865 | |
| Faraday cage | Newport Corporation | VIS-FDC-3600 | |
| Vibration isolation workstation: Vision IsoStation | Newport Corporation | M-VIS3660-RG4-325A | |
| Oximeter Control Unit and sensor: MouseOxPlus, Starr Life Sciences Corp. | WPI | O15001 | |
| Transparent soft nose cone: Microflex Non-Rebreathing Unit with a Rat Nosecone | WPI | EZ-103A |
This article has been published
Video Coming Soon
Source: Kang, S., et al. Concurrent Recording of Co-localized Electroencephalography and Local Field Potential in Rodent. J. Vis. Exp. (2017).
Copyright © 2025 MyJoVE Corporation. All rights reserved