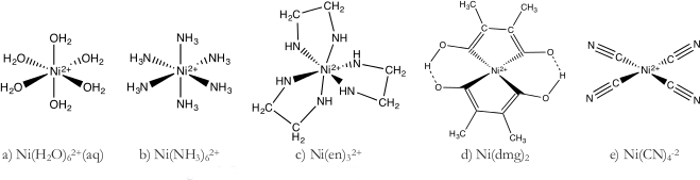
מתחמי כימיה של תיאום
Overview
מקור: המעבדה של ד"ר ניל אברמס — מכללת SUNY למדעי הסביבה ויערנות
מתכות מעבר נמצאות בכל מקום, החל תוספי ויטמינים לאמבטיות electroplating. מתכות מעבר גם מרכיבות את הפיגמנטים בצבעים רבים ומרכיבות את כל המינרלים. בדרך כלל, מתכות מעבר נמצאות בצורה הקטיקטית מכיוון שהן מתחמצןות בקלות, או מאבדות אלקטרונים, ומוקפות בתורמי אלקטרונים הנקראים ליגנדים. ליגנדים אלה אינם יוצרים קשרים יוניים או קוולנטיים עם מרכז המתכת, אלא הם לוקחים על סוג שלישי של קשר המכונה קואורדינטות קוולנט. הקשר הקואורדינט-קוולנטי בין ליגנד למתכת הוא דינמי, כלומר ליגנדים מחליפים ומתאמים מחדש ללא הרף סביב מרכז המתכת. הזהויות של המתכת והליגנד מכתיבות אילו ליגנדים יקשרו באופן מועדף על פני אחר. בנוסף, תכונות צבע ומגנטיות נובעים גם מסוגי המתחמים שנוצרים. תרכובות התיאום שנוצרות מנותחות באמצעות מגוון מכשירים וכלים. ניסוי זה בוחן מדוע כל כך הרבה מתחמים אפשריים ומשתמש בשיטה ספקטרוכימית (צבע וכימי) כדי לסייע בזיהוי סוג קומפלקס הקואורדינציה שנוצר.
Procedure
1. מתחמי ניקל וצבעים
- Ni(H2O)62+ קומפלקס(איור 1a)
- הכן פתרון 1 M של Ni(H2O)62+ על ידי המסת NiSO4 בנפח המתאים של מים.
- לדלל עוד יותר את הפתרון Ni(H2O)62+על ידי הוספת 70 מ"ל של פתרון 1 M ל 1,000 מ"ל של מים deionized.
- חלק את Ni(H2O)62 + בין שבעה 400 מ"ל כות.
- תמיסה ניקל מימי לוקח על צבע ירוק בהיר מאז מים הוא ליגנד שדה חל
Application and Summary
מפיגמנטים ועד אנשים, מתכות מעבר נמצאות בתחומי הכימיה, הביולוגיה, הגיאולוגיה וההנדסה. הבנת ההתנהגות של מתכות מעבר תחת מצבים כימיים שונים יכולה להיות פשוטה כמו ניטור צבע או התנהגות מגנטית. כמעט כל מתכת מעברתלת-ממדית (שורה 4) חיונית לתפקוד הפיזיולוגי, ובכל המקרים, מתכות אלה כבולות על ידי...
References
- Shakhashiri, B. Z.; G. E. Dirreen, G. E; Juergens, F. Color, Solubility, and Complex Ion Equilibria of Nickel (II) Species in Aqueous Solution. J. Chem. Ed. 52 (12), 900-901 (1980).
Tags
Copyright © 2025 MyJoVE Corporation. All rights reserved