פלאק אסאי: שיטה לקביעת טיטר ויראלי כיחידות יוצרות פלאק (PFU)
Overview
מקור: עדדה אנדרסון1, רולף לוד1
1 המחלקה למדעים קליניים לונד, החטיבה לרפואת זיהומים, המרכז הביו-רפואי, אוניברסיטת לונד, 221 00 לונד, שוודיה
וירוסים שמדביקים אורגניזמים פרוקריוטים, הנקראים בקטריופאג'ים או פשוט פאג'ים, זוהו בתחילת המאהה-20 על ידי Twort (1) ו-d'Hérelle (2) באופן עצמאי. מאז הוכרו פאג'ים בזכות ערכם הטיפולי (3) והשפעתם על בני האדם (4), כמו גם על מערכות אקולוגיות גלובליות (5). החששות הנוכחיים עוררו עניין מחודש בשימוש בפאג'ים כחלופה לאנטיביוטיקה מודרנית לטיפול במחלות זיהומיות (6). בעיקרו של דבר כל מחקר פאג 'מסתמך על היכולת לטהר ולכומת וירוסים, הידוע גם בשם טיטר ויראלי. בתחילה תואר בשנת 1952, זו הייתה מטרתו של ההסתה הלוח (7). עשרות שנים והתקדמות טכנולוגית מרובה לאחר מכן, ההסתה של הלוח נותרה אחת השיטות המהימנות ביותר לקביעת טיטר ויראלי (8).
בקטריופאג'ים להתקיים על ידי הזרקת החומר הגנטי שלהם לתאים מארחים, חטיפת המכונות לייצור של חלקיקי פאג 'חדשים, ובסופו של דבר גורם המארח לשחרר ויריונים צאצאים רבים באמצעות תמוגה התא. בגלל גודלם הדק, לא ניתן לצפות בבקטריופאג'ים באמצעות מיקרוסקופיה קלה בלבד; לכן, סריקת מיקרוסקופיית אלקטרונים נדרשת (איור 1). בנוסף, לא ניתן לטפח פאג'ים על לוחות אגר תזונתיים כמו חיידקים, שכן הם זקוקים לתאים מארחים כדי לטרוף.
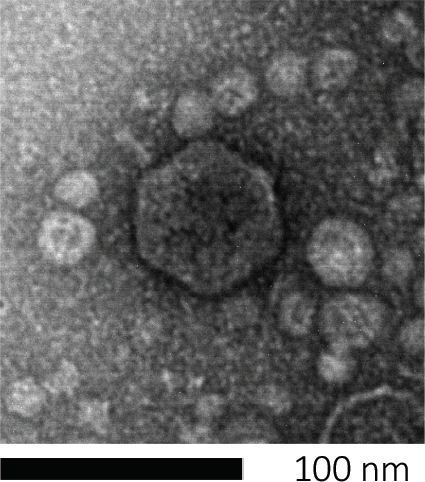
איור 1: ניתן לחקור את המורפולוגיה של בקטריופאג', המודגמת כאן על ידי E. coli phage, באמצעות סריקת מיקרוסקופיית אלקטרונים. רוב הבקטריופאג'ים שייכים ל- Caudovirales (בקטריופאג'ים זנב). זה פאג ' מסוים יש מבנה זנב קצר מאוד וראש icosahedral, הצבתו במשפחה של Podoviruses.
ה-plaque assay (איור 2) מבוסס על שילוב של תאים מארחים, המועדפים על צמיחת שלב היומן, לתוך המדיום. זה יוצר שכבה צפופה, עגום של חיידקים מסוגל לקיים צמיחה ויראלית. פאג' מבודד יכול להדביק, לשכפל בתוך תא אחד ולתייג אותו. עם כל תא lysed, סמוכים מרובים להידבק מיד. מספר מחזורים פנימה, ניתן לראות אזור ברור (פלאק) בלוח העכיר (איור 2B/איור 3A),המציין את נוכחותו של מה שהיה בתחילה חלקיק בקטריופאג' יחיד. לפיכך, ניתן לקבוע את מספר יחידות יצירת הלוח לכל אמצעי אחסון (כלומר PFU/mL) של מדגם, ממספר הלוחות שנוצרו.
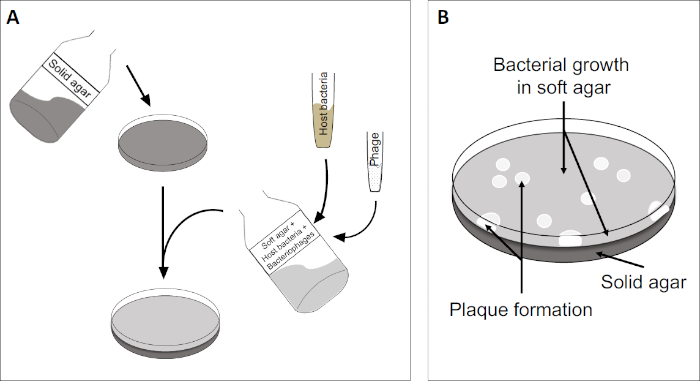
איור 2: בדיקת יחידות יוצרות פלאק (PFU) היא שיטה נפוצה לקביעת מספר הבקטריופאג'ים במדגם. (A) הבסיס של צלחת פטרי סטרילי מכוסה במדיום מודי מתאים, ואחריו תערובת של מדיה רכה, תאים מארחים רגישים ודילול של דגימת הבקטריופאג'. שים לב כי השעיית פאג 'יכול, במקרים מסוימים, גם להיות מפוזר באופן שווה על פני השטח של אגר רך כבר מוצק. (B) צמיחת החיידקים המארחים יוצרת מדשאה של תאים בשכבת אגר העליונה. שכפול בקטריופאג' מייצר אזורים ברורים, או לוחות, הנגרמים על ידי תמה של תאים מארחים.
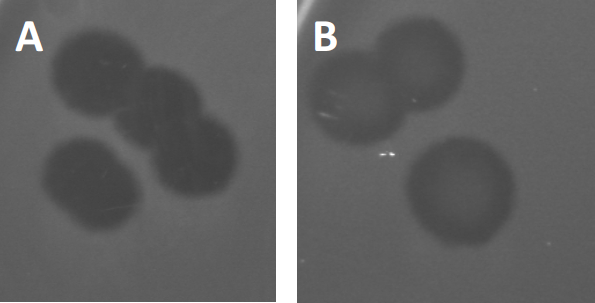
איור 3: תוצאות בדיקות PFU מראות לוחות מרובים שנוצרו על ידי בקטריופאג'ים. בשל תמוגה של תאים מארחים רגישים, ניתן לראות פלאקים כאזורי ניקוי במדשאה החיידקית, עם (A) אישור מלא, או (B) צמיחה מחדש חלקית הנגרמת על ידי יצירת חיידקים עמידים (או אולי על ידי פאג'ים ממוזגים במחזור ליזוזניים).
פאג'ים ממוזגים מסוימים יכולים לאמץ את מה שמכונה מחזור חיים ליזוזני, בנוסף לצמיחה הטיקית שתוארה בעבר. בליסוגניה, הנגיף מניח מצב סמוי באמצעות שילוב של החומר הגנטי שלו בגנום של התא המארח (9), לעתים קרובות מעניק עמידות לזיהומי פאג 'נוספים. זה מתגלה לפעמים דרך ערנות קלה של הלוח (איור 3B). ראוי לציין עם זאת, כי לוחות יכולים גם להיראות מטושטשים עקב צמיחה מחדש של חיידקים שפיתחו עמידות פאג 'ללא תלות בזיהומים פאג 'קודמים.
וירוסים יכולים לצרף, או ספיחה, למגוון מוגבל בלבד של חיידקים מארחים (10). טווחי המארחים מוגבלים עוד יותר על ידי אסטרטגיות אנטי ויראליות תאיות כגון מערכת CRISPR-Cas (11). ההתנגדות/הרגישות כלפי פאג'ים ספציפיים המוצגים על ידי תת-קבוצות חיידקיות שימשה היסטורית לסיווג זני חיידקים לסוגי פאג' שונים (איור 4). למרות שהיעילות של שיטה זו עלתה כעת על טכניקות רצף חדשניות, הקלדת פאג 'עדיין יכולה לספק מידע בעל ערך על אינטראקציות חיידקים-פאג ', למשל, להקל על העיצוב של קוקטייל פאג 'לשימוש קליני.
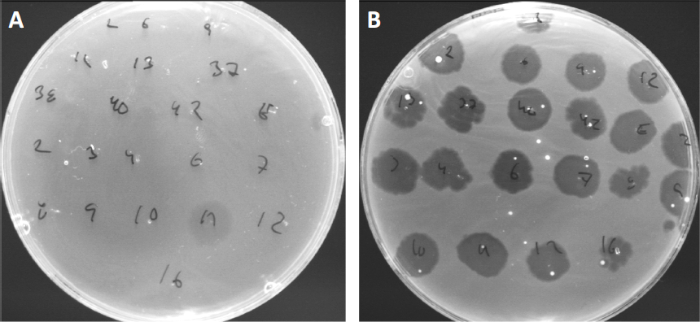
איור 4: רגישות פיג' לזני חיידקים שונים. צלחות אגר רכות עם זן אקנה Cutibacterium (A) AD27 ו (B) AD35, נצפו עם 21 בקטריופאג'ים שונים C. רק פאג' 11 הצליחו להדביק ולהרוג את AD27 בעוד שזן AD35 הראה רגישות כלפי כל הפאג'ים. טכניקה זו, המכונה הקלדת פאג ', יכולה לשמש לחלוקת מינים וזנים חיידקיים לתת-קבוצות שונות המבוססות על רגישות פאג '.
Procedure
1. הגדרת
- לפני תחילת כל עבודה מעורבים חיידקים, ודא כי חלל העבודה מעוקר (למשל ניגב עם 70% אתנול). תמיד ללבוש חלוק מעבדה וכפפות, לשמור על שיער ארוך קשור לאחור, ולהבטיח כי כל הפצעים מוגנים היטב במיוחד.
- בסיום, לעקר את כל המשטחים ולשטוף / לעקר ביסודיות ידיים ופרקי כף היד.
2. פרוטוקול
- הכנת מדיה LB
הערה: בהתאם לזן החיידקי המארח ולבקטריופאג ', מדיום נוזלי אחר עשוי להתאים יותר לפולחן הראשו
Application and Summary
למרות ההתקדמות הטכנולוגית המרובה, תקן הפלאק נשאר תקן הזהב לקביעת טיטר ויראלי (כ- PFU) וחיוני לבידוד אוכלוסיות בקטריופאג 'טהורות. תאים מארחים רגישים מעובדים בשכבה העליונה של צלחת אגר דו שכבתית, ויוצרים מיטה הומוגנית המאפשרת שכפול ויראלי. האירוע הראשוני שבו בקטריופאג' מבודד במחזור חיים ליטי מ...
References
- Twort, F. An investigation on the nature of ultra-microscopic viruses. Lancet. 186 (4814): 1241-1243. (1915)
- d'Hérelle, F. An invisible antagonist microbe of dysentery bacillus. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences. 165: 373-375. (1917)
- Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years After the Discovery of Bacteriophages. Current Microbiology. 74 (2):277-283. (2017)
- Mirzaei MK, Maurice CF. Ménage à trois in the human gut: interactions between host, bacteria and phages. Nature Reviews Microbiology. 15 (7):397. (2017)
- Breitbart M, Bonnain C, Malki K, Sawaya NA. Phage puppet masters of the marine microbial realm. Nature Microbiology. 3 (7):754-766. (2018)
- Leung CY, Weitz JS. Modeling the synergistic elimination of bacteria by phage and the innate immune system. Journal of Theoretical Biology. 429:241-252. (2017)
- Dulbecco R. Production of Plaques in Monolayer Tissue Cultures by Single Particles of an Animal Virus. Proceedings of the National Academy of Sciences of the United States of America. 38 (8):747-752. (1952)
- Juarez D, Long KC, Aguilar P, Kochel TJ, Halsey ES. Assessment of plaque assay methods for alphaviruses. J Virol Methods. 187 (1):185-9. (2013)
- Clokie MRJ, Millard AD, Letarov AV, Heaphy S. 2011. Phages in nature. Bacteriophage. 1 (1):31-45. (2011)
- Moldovan R, Chapman-McQuiston E, Wu XL. On kinetics of phage adsorption. Biophys J. 93 (1):303-15. (2007)
- Garneau JE, Dupuis M-È, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S.. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 468 (7320):67. (2010)
Tags
Skip to...
Videos from this collection:

Now Playing
פלאק אסאי: שיטה לקביעת טיטר ויראלי כיחידות יוצרות פלאק (PFU)
Microbiology
186.7K Views

יצירת טור וינוגרדסקי: שיטה להעשרת המינים המיקרוביאליים בדוגמת משקעים
Microbiology
130.2K Views

דילול סדרתי ו ציפוי: ספירה מיקרוביאלית
Microbiology
317.3K Views

תרביות העשרה: מיקרואורובים אירוביים ואנאירוביים פולחניים על מדיות סלקטיביות ודיפרנציאליות
Microbiology
132.4K Views

תרביות טהורות ו ציפוי פסים: בידוד מושבות חיידקיות בודדות מדגם מעורב
Microbiology
166.6K Views

ריצוף rRNA 16S: טכניקה מבוססת PCR לזיהוי מינים חיידקיים
Microbiology
189.8K Views

עקומות צמיחה: יצירת עקומות צמיחה באמצעות יחידות יוצרות מושבה ומדידות צפיפות אופטית
Microbiology
298.1K Views

בדיקת רגישות אנטיביוטית: בדיקות אפסילומטר לקביעת ערכי המיקרופון של שתי אנטיביוטיקות והערכה של סינרגיה אנטיביוטית
Microbiology
94.2K Views

מיקרוסקופיה וכתמים: גרם, כמוסה וכתמים אנדוספור
Microbiology
364.3K Views

טרנספורמציה של תאי E. coli באמצעות הליך סידן כלוריד מותאם
Microbiology
87.2K Views

הטיות: שיטה להעברת עמידות אמפיצ'ין מתורם לנמען E. coli
Microbiology
38.5K Views

טרנסדוקציה של פאג': שיטה להעברת עמידות לאמפיצ'ילין מתורם לנמען E. coli
Microbiology
29.2K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved
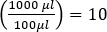 (כלומר)
(כלומר)