Method Article
Micro-Ultrasound Guided Transperineal Prostate Biopsy: A Clinic-Based Procedure
In This Article
Summary
This manuscript outlines the steps for performing a micro-ultrasound-guided transperineal prostate biopsy under local anesthesia.
Abstract
Prostate cancer is the most common solid malignancy in men and requires a biopsy for diagnosis. This manuscript describes a freehand micro-ultrasound guided transperineal technique performed under local anesthesia, which maintains accuracy, keeps patients comfortable, has low adverse events, and minimizes the need for disposables. Prior micro-ultrasound-guided transperineal techniques required general or spinal anesthesia. The key steps described in the protocol include (1) the placement of the local anesthesia, (2) micro-ultrasound imaging, (3) and the visualization of the anesthetic/biopsy needle while uncoupled from the insonation plane. A retrospective review of 100 patients undergoing this technique demonstrated a 68% clinically significant cancer detection rate. Pain scores were prospectively collected in a subset of patients (N = 20) and showed a median procedural pain score of 2 out of 10. The 30 day Grade III adverse event rate was 3%; one of these events was probably related to the prostate biopsy. Overall, we present a simple, accurate, and safe technique for performing a micro-ultrasound-guided transperineal prostate biopsy.
Introduction
Prostate cancer is the most common solid malignancy among American men, with 268,000 projected diagnoses in 20221. The diagnosis of prostate cancer requires a prostate biopsy, which is traditionally performed by passing a biopsy needle through the rectal mucosa (transrectal) into the prostate. The needle guidance follows a systematic "blind" template, as conventional ultrasound cannot differentiate cancer from benign tissue2. In the United States, over 1 million such biopsies are performed annually3,4.
Over the last decade, two significant advances in imaging and techniques have improved safety and accuracy. First, increasing the use of transperineal biopsies to avoid the rectal mucosa has decreased the risk of sepsis without needing antibiotics5,6. Second, the use of MRI and micro-ultrasound (Micro-US) have improved cancer detection rates compared to biopsy with conventional (5 MHz) ultrasound guidance5,7,8,9.
Micro-US utilizes 29 MHz acoustic waves, improved piezoelectric crystal density, and novel wave processing to achieve a spatial resolution of 70 µm compared to around 200 µm for a conventional ultrasound8. A grading system using a 5-point Likert scale (Prostate Risk Identification Using Micro-Ultrasound, PRI-MUS), is used to quantify the risk of prostate cancer in micro-ultrasound lesions10. Biopsy core and PRI-MUS lesion locations are tracked relative to the midline using an accelerometer located in the handle of the Micro-US probe (ExactImaging, Markham, ON). The ability to track biopsies enables the 3D reconstruction of prostate cancer locations. Precision cancer treatments such as focal therapy and radiation boosting are enabled through the spatial information gained by tracked cores.
To date, no published techniques enabling micro-ultrasound-guided transperineal prostate biopsy under local anesthesia can also retain the spatial orientation of the cores and lesions. This manuscript aims to delineate such a technique.
Protocol
The methods described are based on experience at the University of Florida (UF). The protocol and acquisition of data were approved by the University Institutional Review Board (IRB). Indications for prostate biopsy included a suspicious digital rectal exam (DRE), prostate-specific antigen (PSA) elevation, or another biomarker abnormality (i.e., 4K, ExoDx). The protocol is described for a right-handed surgeon.
1. Micro-ultrasound probe preparation
- Disinfect the probe as per the micro-ultrasound "System Operation and Safety Manual"11.
- Place around 20 mL of acoustic gel at the end of the probe cover, and remove bubbles. Place a needle guide on the probe, and place the probe cover over the probe (Figure 1A).
NOTE: Bubbles are removed by rotating the probe cover with lube as with a foxtail toy. The centrifugal force displaces the lubrication distally while moving the bubbles proximally. - Place a rubber band around the probe neck below the piezoelectric crystals to prevent gel leakage.
- Set up the biopsy equipment as per the surgeon's preference.
NOTE: The setup used in this study is demonstrated in Figure 1B.
2. Patient positioning and anesthesia
- Position the patient in the lithotomy position with the anus at the edge of the bed. Provide head support and a genital drape to improve comfort and privacy (Figure 1C).
NOTE: At UF, stirrups for lithotomy positioning are preferred. - Place 10 mL of 2% viscous lidocaine gel into the rectum as previously described12.
- Ensure patient comfort and the preparation of the necessary materials during this time.
- Adjust the bed height so that the anus is at the level of the surgeon's elbow.
- Instruct the patient to elevate their scrotum and expose the perineum (Figure 1C).
- Using acoustic gel, perform a digital rectal exam. Note palpable abnormalities for staging and biopsy targeting.
- Change gloves, and sterilize the perineal skin with betadine or chlorhexidine from the base of the scrotum to below the anus.
- Anesthetize the perineal soft tissue at the 10 o'clock and 2 o'clock positions, starting around 10-15 mm from the anus (Figure 1D).
NOTE: Begin with a 2 inch 25 G needle containing 1% lidocaine with 1:100,000 epinephrine and 8.4% sodium bicarbonate solution mixed in a 1:10 ratio (1 mL of NaHCO3: 10 mL of lidocaine). This is, hereafter, referred to as "lidocaine." - Insert the needle parallel to the rectum, and inject lidocaine as the needle is advanced. Place around 3 mL of lidocaine in the soft tissue between the skin and Colles' fascia and 2 mL between the Colles' fascia (Figure 2A).
NOTE: For efficiency, the above step is performed in a blind fashion; however, early in one's experience, consider utilizing ultrasound guidance as patient anatomy differs with pelvic shape, gluteal musculature, and obesity.
3. Micro-ultrasound diagnosis
- Lubricate the probe, gently insert it into the rectum, and advance until the mid-prostate is centered on the viewing monitor.
NOTE: Follow the contour of the sigmoid by directing the probe tip downward (toward the sacrum) once beyond the anal sphincter. Using this technique, the bladder base can be visualized with minimal discomfort. Failing to direct the probe downward will cause pain. - Optimize the micro-ultrasound image settings.
- Grasp the imaging probe in the left (non-dominant) hand at the thickest portion of the probe. Pitch and lift the probe upward so that the base of the prostate receives the greatest pressure from the piezoelectric crystals. The pitching and lifting motion will compress the rectum and optimize visualization (Figure 1C).
- Adjust the time-compensated gain (TCG) sliders into a gentle reverse "C" or "J" shape until there is a uniform gray scale across the image.
- Adjust the master gain dial so that the lateral edges of the prostate are well visualized yet the center of the prostate is not too bright.
NOTE: The lateral prostate is acoustically darker than the mid gland as fewer reflected ultrasound waves return at an angle. - Ensure no bubbles or rectal mucus are causing a shadow.
NOTE: At UF, the default settings are utilized for dynamic range, depth, and focus; however, these can be adjusted as per surgeon preference. - Select an image preset (Small, Medium, Large, and Extra-Large) that enables the visualization of the prostate with minimal anterior prostatic fat.
NOTE: Details of the image optimization and Micro-US operation are available in the Micro-US "System Operation and Safety Manual"11.
- Perform a prostate volume calculation by pressing the Dual/Transverse button, performing a prostate sweep, and then measuring the sagittal length and reconstructed axial height and width.
- Rotate the probe until the urethra is visualized. The visualization of the urethra is best at the bladder, neck, and prostate apex. Place the probe parallel to the midline so the bladder neck and urethra are visible in the same plane (yaw neutral).
- Press the Angle Reset on the Micro-US touch screen to zero the probe accelerometer.
- Place the right hand at the proximal portion of the probe where the probe meets the electrical cord. The left hand slides under the probe neck, supporting the upward pitch position (Figure 1C).
- Tell the patient to hold still; in one motion, sweep from the right lateral prostate border (clockwise) through the prostate to the left edge, taking ~25 s. Press the Cine Loop button to record the sweep.
NOTE: Separate sweeps of the apex and base are often required for prostates larger than 50 mL. It is best practice to record a sweep that visualizes the transition zone and a sweep on the Small setting to visualize the peripheral zone.
4. Diagnostic evaluation
- Evaluate the transition zone for PRI-MUS lesions.
- Once the transition zone has been evaluated, switch to the Small image setting, and evaluate the peripheral zone for PRI-MUS lesions.
NOTE: Consider practicing using the free Micro-US diagnostic library to gain experience with identifying and grading PRI-MUS lesions11. - Note the location of the encountered regions of interest (ROI) by recording the center angle and the angles at which the ROI is first and last visible.
NOTE: At UF, these angles are called out by the surgeon and recorded in the operative notes by staff in the biopsy suite. - If utilizing fusion software, load a pre-biopsy annotated MRI, and press the Mid-line button while visualizing the urethra (Figure 2B).
5. Prostate anesthesia
- Using a 6 inch 20 G needle, puncture the skin at the previously anesthetized biopsy tract, and advance the needle to the levator ani under Micro-US visualization (Figure 2A).
- Place 3 mL of the local anesthetic into the levator ani muscle complex (Figure 2A).
- Advance the 20 G needle into the peri-apical triangle (bounded by the urethra medially, the levator ani laterally, and the Denonvilliers' fascia posteriorly). Inject 5-7 mL of lidocaine into the peri-apical triangle (Figure 2A).
NOTE: The lateral aspect of the dorsal venous complex (DVC) drapes into the periapical triangle; thus, aspirate before the lidocaine injection. Appropriate anesthesia will hydrodissect between the prostate capsule and Denonvilliers' fascia.
6. Prostate biopsy
- Insert a 14 G angiocatheter or 15 G metal coaxial needle (access needle) through the previously anesthetized tract to within 5-10 mm of the prostate apex. Pass the access needle above the anal sphincter and through or medial to the levator ani. Customize the distance between the access needle and the rectum according to the ROI location.
- Sample the entire prostate using a needle location 5 mm above the rectum and 15°-20° from the mid prostate plane where the urethra is visible (Figure 1D, 10 o'clock and 2 o'clock positions).
- Rotate the probe so that the insonation plane visualizes the access needle, and advance the biopsy gun until the biopsy needle tip becomes visible at the end of the access needle.
- Rotate the probe to visualize the biopsy target (either the ROI or systematic core), and then rotate back to visualize the biopsy needle tip. Adjust the needle trajectory toward the target by adjusting the biopsy gun external to the patient.
- Advance the needle under vision, and complete small 3°-10° probe rolls (micro rolls) to guide the needle tip into the intended target. Only advance the biopsy needle while visualizing the tip to prevent overshooting the intended target.
NOTE: It is necessary to withdraw the biopsy needle if making larger movements toward a target. In this technique, the probe is kept parallel to the urethra rather than parallel to the needle ("yawing"). Preserving the biopsy angle relative to the urethra allows an accurate reconstruction of the cancer location and maximizes patient comfort. The most challenging portion of this technique is visualizing the needle when uncoupled from the insonation plane. In our experience instructing residents, fellows, and other physicians, the muscle memory required to implement the technique requires around 3 h of practice. Consider practicing on a prostate phantom or simulator13 to build the requisite muscle memory before a biopsy. Performing this technique initially on an anesthetized patient will ease the transition to a clinic-based practice. - Before obtaining a biopsy sample, roll it to the urethra, and ensure the angle is within 1°-2° of the 0° position. Record the angle position for each biopsy throw by capturing a frame or cine loop.
NOTE: By convention, right-sided (counterclockwise) rotation is indicated by a positive angle, while left-sided (clockwise) rotation is characterized by a negative angle. - Begin by obtaining three biopsy cores distributed through the ROI (Figure 2C).
- Consider performing a transperineal systematic biopsy (Figure 3). For efficiency, begin systematic biopsies ipsilateral to the ROI. If the ROI is anterior, lower the coaxial needle to improve the systematic biopsy sampling of the peripheral zone.
NOTE: This study performed a 14-core systematic biopsy (Figure 3). A systematic biopsy can likely be skipped in these instances: 1) systematic cores overlapping a previously sampled ROI, and 2) small prostates where a single throw samples the apex and base. Utilizing this template, apical biopsies are obtained by beginning the throw at the apical prostate capsule. Base biopsies are obtained by starting the throw in the mid gland.
7. Procedure end
- After acquiring all the biopsies, remove the coaxial needle and ultrasound probe.
- Hold pressure on the perineum with a towel for 1-5 min until puncture site hemostasis is achieved.
- Assist the patients to a seated position and, after 1 min, a standing position.
- Counsel the patients to expect transient hematuria and hematospermia.
- Prescribe patients with baseline restricted urinary stream alpha-blockers to be filled as needed.
Results
A retrospective analysis of our prospectively maintained database from September 2021 to June 2022 (IRB202200022) is described. Clinically significant prostate cancer (csPCa) was considered ≥Grade Group 2 (Gleason 3 + 4 = 7) prostate cancer. The cancer detection rate (CDR) was calculated as ≥GG2/total patients. Pain scores were evaluated on a 1-10-point Likert scale at baseline, at local anesthesia placement, at rectal probe insertion, and during the biopsy. Adverse events were discussed with patients at 2 weeks following the procedure, and chart review-captured events were documented within 30 days of the biopsy. Adverse events were graded using the CTEP (Cancer Therapy Evaluation Program) grade and attribution guide.
We identified 100 patients who underwent Micro-US guided, clinic-based transperineal biopsy under local anesthesia. In this study, the per-patient CDR was 68%. The CDR varied depending on each lesion's PI-RADS (Prostate Image Reporting and Data System) and PRI-MUS scores (Table 1).
Pain scores were available amongst a later subset of patients (N = 20). The median (IQR) pain scores were 0 (0, 1) at baseline, 2 (1, 4) during the administration of local anesthesia, 2 (1, 5) during the transrectal probe placement, and 2 (0, 5) during the prostate biopsy.
Adverse events were relatively rare, with a 3% incidence of Grade III adverse events (Table 2). There were no Grade IV or Grade V events. The Grade II events primarily involved the new prescription of alpha-blockers to alleviate a slowing urinary stream. As mentioned above, the patients were screened for symptoms before the biopsy. Grade III events involved the hospitalization of three patients. As per the CTEP attribution guide, one hospitalization due to symptomatic hypotension was probably related to the prostate biopsy secondary to lidocaine absorption. The patient experienced a spontaneous resolution of symptoms within 12 h of monitoring. Two additional patients were hospitalized (mechanical fall and altered mental status) within 30 days of the biopsy. They were incidentally found to have non-septic and non-symptomatic UTIs, possibly related to the biopsy. Aside from these specific patients mentioned, there were no sepsis, prostatitis, cystitis, or other infection cases.
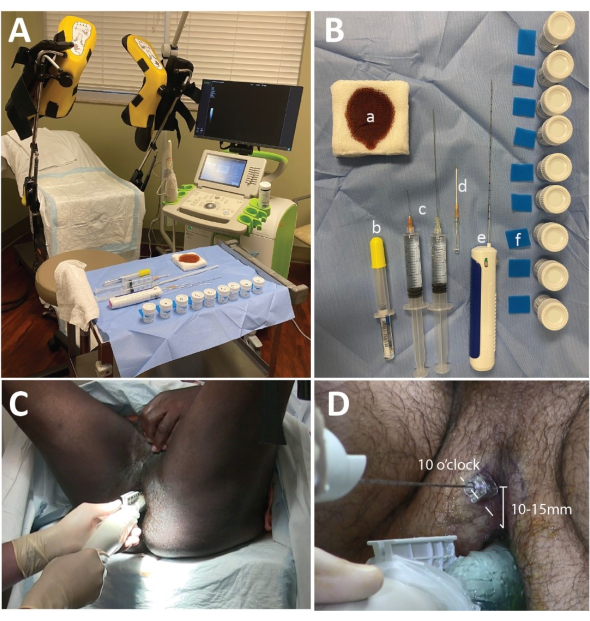
Figure 1: Micro-ultrasound setup, biopsy tray, and needle placement. (A) The procedure room setup with the ExactVu Machine placed on the surgeon's right. (B) Biopsy table including (a) betadine-soaked 4 cm x 4 cm gauze pads, (b) 10 mL of 2% lidocaine gel, (c) 1% lidocaine with 1:100,000 epinephrine distributed with 2 mL of 8.4% sodium bicarbonate solution (1 mL of NaHCO3: 10 mL of lidocaine) in 2x 20 mL syringes with a 2 inch 25 G needle and a 6 inch 20 G needle, (d) a 14 G angiocath or coaxial metal needle, (e) a biopsy gun, (f) foam biopsy pads, and formalin containers. (C) The hand position for performing the diagnostic sweep. Note that the left hand supports the upward pressure on the prostate, while the right hand rotates the probe. (D) The coaxial needle or angiocatheter position: 10 o'clock for the right-sided prostate, and 2 o'clock for the left-sided prostate (not shown). Please click here to view a larger version of this figure.

Figure 2: Demonstration of the prostate anatomy and biopsy. (A) Visualization of the levator ani and periapical triangle; both are important structures to anesthetize for the successful implementation of this technique. (B) Demonstration of a PRI-MUS 5 lesion, anatomically concordant with the pre-biopsy MRI. (C) Visual confirmation of the biopsy needle traversing the target. Please click here to view a larger version of this figure.
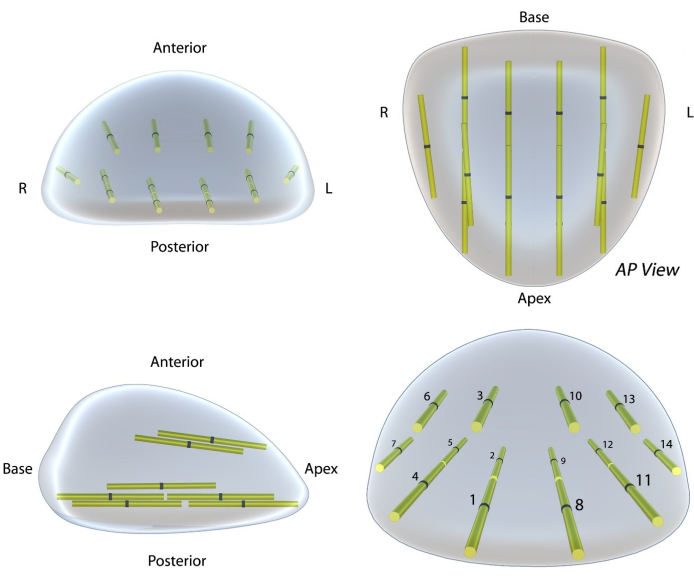
Figure 3: Systematic biopsy template for the proposed technique. This template is based on the template available in the Michigan Urologic Surgery Improvement Collaborative (MUSIC)14. However, this template is an improvement in three regards. (i) Increased anterior zone sampling (4 cores versus 2 cores), (ii) Greatly improved visual display of the core location using a 3D model, and (iii) modified core nomenclature to improve usability. In this protocol, systematic biopsies are taken after the ROI biopsy within imaging negative (PRI-MUS ≤ 2) tissue. Please click here to view a larger version of this figure.
| PI-RADS 5 | PI-RADS 4 | PI-RADS 3 | |
| PRI-MUS 5 | 95% (n = 20) | 80% (n = 15) | 100% (n = 1) |
| PRI-MUS 4 | 100% (n = 1) | 69% (n = 13) | 75% (n = 4) |
| PRI-MUS 3 | 0% (n = 0) | 33% (n = 3) | NA |
Table 1: Detection of clinically significant prostate cancer stratified by PI-RAD and PRI-MUS scores. Clinically significant prostate cancer detection rate (CDR) among patients with both MRI and micro-ultrasound available at the time of biopsy. The CDR is stratified by the PI-RADS (column) and PRI-MUS scores (row). The number of patients meeting these criteria is recorded in parentheses.
| CLAVIEN DINDO GRADE | INCIDENCE |
| GRADE I | 4% (n = 4) |
| GRADE II | 5% (n = 5) |
| GRADE III | 3% (n = 3) |
| GRADE IV | 0% (n = 0) |
| GRADE V | 0% (n = 0) |
Table 2: Percentage of patients experiencing adverse events by grade. Percentage of patients with an adverse event within 30 days of the prostate biopsy. Note that Grade III adverse events were hospitalizations for non-septic events, one of which was probably related to the biopsy and two of which were possibly associated with the biopsy.
Discussion
This manuscript details the procedure and results for Micro-US guided, freehand, transperineal prostate biopsy under local anesthesia. This is the first description of a technique that preserves the biopsy core location and patient comfort. In our experience, the procedure is accurate, well tolerated, and has minimal adverse events.
Notably, there were no reported cases of sepsis or prostatitis despite the lack of prophylactic antibiotics. The results from the sample used here corroborate the findings and complication rate of the NORAPP trial, which did not find a benefit of prophylactic antibiotics in transperineal biopsy6.
The critical steps of the procedure are as follows. The disinfection and anesthesia noted in step 2.6 and section 5 are the key components for reducing the infection rates by traversing the perineum rather than the rectal wall while maintaining patient comfort. Section 4 delineates the ROIs for biopsy targets, allowing for fewer cores to be taken while effectively sampling the problematic areas of the prostate.
Transperineal biopsy has gained popularity compared to alternative methods given the zero rates of sepsis15,16, the accuracy of sampling anterior tumors, the antibiotic stewardship, and guideline recommendations17,18. Simultaneously, Micro-US is being recognized as an accurate cancer imaging modality, simple fusion platform, and independent imaging biomarker9,19. Prior Micro-US guided transperineal techniques reported an excellent CDR of 42%; however, this technique utilized a needle guide to align the needle and insonation plane. While using a guide improves the learning curve, it likely confines biopsy to the operating room to allow for the use of general anesthesia, as each biopsy puncture enters through a new site20. We report a CDR of 68%, demonstrating that this free-hand technique has comparable accuracy to the use of a needle guide.
Our technique's accuracy, tolerability, and minimal adverse events must be interpreted within certain limitations. Approximately 3 h of hands-on practice on a simulator or phantom is required to locate and direct the biopsy needle. Additional time may be required to learn the Micro-US image interpretation. Performing targeted plus systematic biopsies can result in 17 or more biopsy cores and require up to 30 min of procedure time. While we report excellent cancer detection rates, the success of this technique partly relates to our practice of omitting the prostate biopsy in patients with a <15% calculated risk of csPCa21. Finally, the systematic biopsy template used in this study samples the highest-risk areas of the prostate but has never been compared to other transperineal biopsy templates. Despite these limitations, this technique has several advantages, most notably the ability to perform the procedure in a clinic-based environment. Additionally, the position tracking of the micro-US probe allows for the 3D reconstruction of the cancer locations for use when performing surgical extirpation, radiation boosting, or focal therapy.
In conclusion, Micro-US represents a recent improvement in prostate biopsy. We demonstrate a transperineal approach that can be implemented in the clinic under local anesthesia. While further evaluation and improvements to the diagnostic capacity of Micro-US are warranted, surgeons adopting this technique will find it simple to implement, accurate, and safe.
Disclosures
None.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| 14 ga Angiocatheter | BD Angiocath | BD382269 | |
| Aquasonic | Parker Labs | Aquasonic 100 | |
| Biopsy Needle | Bard | MaxCore | |
| Biopsy Sponge 2 mm x 25.4 mm x 30.2 mm | McKesson | 1019107 | |
| ExactVu Micro-Ultrasound Machine | ExactImaging | ||
| ExactVu Micro-Ultrasound Probe (EV29L) | ExactImaging | ||
| Guaze Sponge McKesson Cotton 12-Ply 4'' x 4'' | McKesson | 762703 | |
| Hypodermic Needle 25 G 1.5 inch | McKesson | 42142523 | |
| Lidocaine 1% with Epinephrine 1:100,000 | NA | ||
| Lidocaine 2% Gel, 20 mL | URO-Jet | 76329301505 | |
| Probe Cover | ExactImaging | ||
| Skin Prep Solution betadine (10%) | McKesson | 1073829 | |
| Spinal Needle 20 G, 6 inch | McKesson | 992546 | |
| TruGuide | Bard | C1616A |
References
- Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 72 (1), 7-33 (2022).
- Onur, R., Littrup, P. J., Pontes, J. E., Bianco, F. J. Contemporary impact of transrectal ultrasound lesions for prostate cancer detection. The Journal of Urology. 172 (2), 512-514 (2004).
- Loeb, S., Carter, H. B., Berndt, S. I., Ricker, W., Schaeffer, E. M. Complications after prostate biopsy: Data from SEER-Medicare. The Journal of Urology. 186 (5), 1830-1834 (2011).
- Borghesi, M., et al. Complications after systematic, random, and image-guided prostate biopsy. European Urology. 71 (3), 353-365 (2017).
- Pradere, B., et al. Nonantibiotic strategies for the prevention of infectious complications following prostate biopsy: A systematic review and meta-analysis. The Journal of Urology. 205 (3), 653-663 (2021).
- Jacewicz, M., et al. Antibiotic prophylaxis versus no antibiotic prophylaxis in transperineal prostate biopsies (NORAPP): A randomised, open-label, non-inferiority trial. The Lancet. Infectious Diseases. 22 (10), 1465-1471 (2022).
- Ahmed, H. U., et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. The Lancet. 389 (10071), 815-822 (2017).
- Kasivisvanathan, V., et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. The New England Journal of Medicine. 378 (19), 1767(2018).
- Sountoulides, P., et al. Micro-ultrasound-guided vs multiparametric magnetic resonance imaging-targeted biopsy in the detection of prostate cancer: A systematic review and meta-analysis. The Journal of Urology. 205 (5), 1254-1262 (2021).
- Ghai, S., et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: Creation of the micro-ultrasound protocol for prostate risk identification. The Journal of Urology. 196 (2), 562-569 (2016).
- Exact Imaging E-learning. Exact Imaging. , Available from: https://elearn.exactimaging.com/learn (2022).
- Siddiqui, E. J., Ali, S., Koneru, S. The rectal administration of lignocaine gel and periprostatic lignocaine infiltration during transrectal ultrasound-guided prostate biopsy provides effective analgesia. Annals of the Royal College of Surgeons of England. 88 (2), 218-221 (2006).
- Zhang, Z., et al. Attitude is everything: Keep probe pitch neutral during side-fire prostate biopsy. A simulator study. BJU International. 128 (5), 615-624 (2021).
- Prostate Biopsy. Michigan Urological Surgery Improvement Collaborative (MUSIC). , Available from: https://musicurology.com/programs/prostate/transperinealbiopsy/ (2022).
- Sigle, A., et al. Safety and side effects of transperineal prostate biopsy without antibiotic prophylaxis. Urologic Oncology: Seminars and Original Investigations. 39 (11), (2021).
- Grummet, J. P., et al. Sepsis and "superbugs": Should we favour the transperineal over the transrectal approach for prostate biopsy. BJU International. 114 (3), 384-388 (2014).
- Pilatz, A., et al. European association of urology position paper on the prevention of infectious complications following prostate biopsy. European Urology. 79 (1), 11-15 (2021).
- Meyer, A. R., et al. Transperineal prostate biopsy improves the detection of clinically significant prostate cancer among men on active surveillance. The Journal of Urology. 205 (4), 1069-1074 (2021).
- Avolio, P. P., et al. The use of 29 MHz transrectal micro-ultrasound to stratify the prostate cancer risk in patients with PI-RADS III lesions at multiparametric MRI: A single institutional analysis. Urologic Oncology: Seminars and Original Investigations. 39 (12), 1-7 (2021).
- Rodríguez Socarrás, M. E., et al. Prostate mapping for cancer diagnosis: The Madrid protocol. Transperineal prostate biopsies using multiparametric magnetic resonance imaging fusion and micro-ultrasound guided biopsies. The Journal of Urology. 204 (4), 726-733 (2020).
- Kinnaird, A., et al. A prostate cancer risk calculator: Use of clinical and magnetic resonance imaging data to predict biopsy outcome in North American men. Canadian Urological Association Journal. 16 (3), 161-166 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved