抗生物質感受性試験:2つの抗生物質のMIC値を決定し、抗生物質の相乗効果を評価するエプシロメーター試験
概要
ソース: アンナ・ブレックバーグ1, ロルフ・ルード1
1臨床科学ルンド, 感染医学の部門, ルンド大学生物医学センター, 221 00 ルンドスウェーデン
微生物が抗生物質耐性をどのように進化させるかを理解する上で、抗生物質と細菌の相互作用に関する知識が重要である。1928年、アレクサンダー・フレミングは、細胞壁再生を妨げ、抗菌機能を発揮する抗生物質であるペニシリンを発見しました(1)。その後、細菌のDNA複製やタンパク質翻訳を阻害する薬剤を含む、多様な作用機序を持つ他の抗生物質が発見された。しかし、近年、新しい抗生物質は開発されていない。現在の抗生物質に対する耐性が高まり、効果的に治療できない重篤な感染症が生じています(2)。ここでは、細菌集団における抗生物質耐性を評価するいくつかの方法について説明する。これらの方法のそれぞれは、使用される抗生物質の作用機序にかかわらず、細菌死が測定された結果であるため、機能する。抗生物質耐性は、病院の設定を通じて特に急速に広がるだけでなく、社会全体に広がっています。このような抵抗手段を調べるために、エプシロメータ試験(E検定)やブロス希釈試験(3)を含む様々な方法が開発されている。
E検定は確立された方法であり、微生物の目に見える成長を阻害する抗菌剤の最低濃度である最小限の阻害濃度(MIC)データを定量する費用対効果の高いツールです。使用される細菌株および抗生物質によって、MIC値はサブμg/mLから>1000 μg/mL(4)の間で変化する可能性がある。E検定は、あらかじめ定義された抗生物質勾配を含むプラスチックストリップを使用して行われ、これはμg/mLでMIC読み取りスケールが刻印されています。このストリップは、接種された寒天プレートに適用される場合、寒天マトリックス上で直接転送されます。インキュベーション後、細菌の増殖が防止されるにつれて、対称的な楕円阻害ゾーンがストリップに沿って見える。MIC は、楕円がストリップと交差する終点である阻害の領域によって定義されます。MICを決定するもう一つの一般的な方法は、マイクロブロス希釈法である。マイクロブロス希釈は、接種細菌を含むブロス培地に添加された抗菌剤の異なる濃度を組み込む。インキュベーション後、MICは目に見える成長を防ぐ抗生物質の最も低濃度として定義される(5)。また、定量的な方法であり、いくつかの細菌に適用することができます。この方法の欠点は、試薬の濃度と実験に必要な試薬の数が多い場合にエラーが発生する可能性があることです。抗生物質耐性の測定は、臨床および研究の両方の観点から不可欠であり、耐性を調査するこれらのインビトロ法について以下に説明します。
特定の細菌に対する抵抗性のプロファイルは、患者が併用治療と単一療法のどちらから利益を得るかを決定するために抗生物質治療を最適化するために適用することができる。一度に複数の抗生物質を使用するためには、互いの相互作用を知ることが不可欠であり、それらが添加剤、相乗的、または拮抗効果を有するかどうかを知ることが不可欠です。抗生物質の関節効果が等しい用量で与えられた個々の抗生物質の効力に等しい場合、添加効果が見られます。一方、抗生物質間の相乗効果は、薬剤が単独で与えられる場合よりも抗生物質の関節効果がより強力である場合に存在する(6)。抗菌治療の組み合わせを適用することは、このように個々の抗生物質治療の効果を高めるために抗菌性の発生を回避するために使用される(7)。抗菌性の知識は、抗菌の組み合わせの不必要な使用を防ぐためにも重要です。E検定方法論は、異なる抗菌剤間の可能な相乗効果と拮抗作用を決定するための簡単かついくつかの方法を提供する。抗生物質耐性病原体の増殖に直面するためには、特定の抗生物質の相乗的および拮抗的なメカニズムの可能性に関する知識が重要であり、臨床的有効性および多剤耐性との闘いをもたらす。
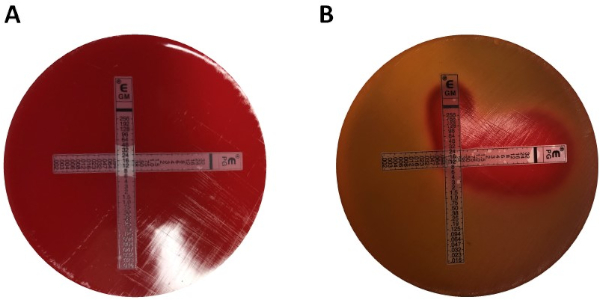
E検定を用いたシナジーの決定は、クロステストと非クロステストの2つの大きなアプローチに分けられます。いずれの相乗効果テストも、個々のMIC値に関する以前の知識に依存していますが、この2つのアプローチは方法論と概念的アプローチにおいて若干異なります。非クロスシナジー試験では、試験対象のペアの最初の抗生物質が細菌に接種された寒天板上に置かれる。最初のストリップからの抗生物質がプレートを注入することを許可した後(例えば、1時間後)、ストリップが除去され、2番目の抗生物質を含む新しいストリップが最初のとまったく同じ場所に置かれ、各otの上に2つの個々のMIC値を置くことを確認します。彼女。得られた阻害ゾーンは、上記のように分析することができ、式1に基づいて計算された相乗効果。
式 1 - 分数阻害濃度 (FIC)
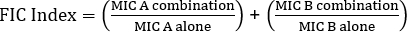
値 >0.5 は相乗効果を示します。
分析しやすいプレートで試験官に報酬を与える一方で、ストリップの変更や実験ごとに2枚のプレートを使用する必要性により、多少手間と時間がかかります。代わりに、クロステストが使用されることがよくあります。2つの異なるE検定ストリップを互いに上に追加する代わりに(最初の除去後)、両方が同時に配置されますが、クロス(90° 角度)の形で、以前に決定された2つのMIC値が90°角度を形成します。このアプローチでは、シナジーテストごとに1つのプレートのみが必要であり、作業が少ないため、分析が少し難しいにもかかわらず、好ましい選択になります。組み合わせた抗生物質アプローチにおける新しいMIC値は、修飾阻害ゾーンとして視覚化することができ、その後、相乗効果は式1によって決定することができる。
角度)の形で、以前に決定された2つのMIC値が90°角度を形成します。このアプローチでは、シナジーテストごとに1つのプレートのみが必要であり、作業が少ないため、分析が少し難しいにもかかわらず、好ましい選択になります。組み合わせた抗生物質アプローチにおける新しいMIC値は、修飾阻害ゾーンとして視覚化することができ、その後、相乗効果は式1によって決定することができる。
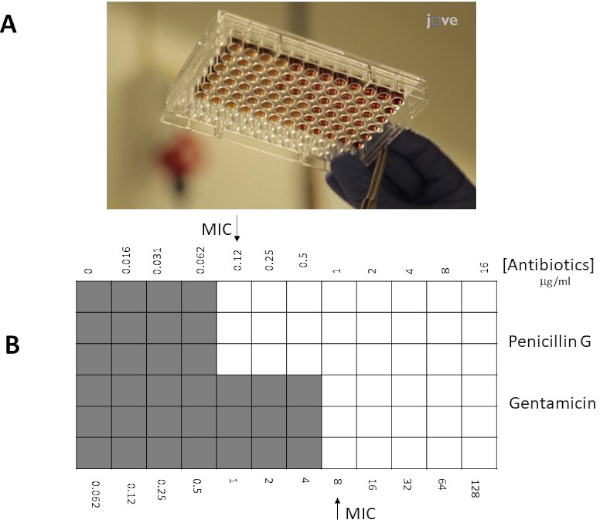
寒天プレートアプローチを使用する代わりに、マイクロブロスアプローチは、多くの場合、その柔軟性が高いため優先することができます(例えば、E検定ストリップの限界外の抗生物質の特定の濃度を選択する能力)。また、固相内の解離に依存しない液体溶液中の抗生物質の均一な分布のために、マイクロブロス検査はより敏感であることが示唆される。.96ウェルマイクロプレートのウェルは、一定数の細菌(106 cfu/mL:細菌濃度はOD600nm測定、濁り基準、または10倍の細菌連続希釈からめっきサンプルを広げることによって推定することができる)で接種され、および異なる希釈の抗生物質が井戸に加えられる。同様に、E検定ストリップにMICは、細菌の目に見える増殖を阻害する抗生物質の最も低濃度との交点(ウェル/スポット)として決定される。
実験的な目的
- 以下のプロジェクトは、ペニシリンGと連鎖球菌群GのゲンタマイシンのMIC値を、E検定およびマイクロブロス希釈の2つの異なる方法によって決定する戦略について説明する。E検定では、ストレプトコッカス群Gに接種したミューラー・ヒントン寒天プレートをペニシリンGおよび/またはゲンタマイシンの勾配ストリップと組み合わせて使用した。50%溶解した馬の血液と20mg/mL β-NADを用いたMH-ブロスは、マイクロブロスアプローチでストレプトコッカス群Gと共に可溶性抗生物質と共に使用した。
材料
- 血液寒天プレート上の細菌コロニー、4°Cで保存<7日
- 血の寒天プレート
- 0.5 マクファーランド標準
- 1% バクル2
- 1% H2SO4
- 生理生理管(2 mL)
- コットンチップアプリケーター
- ミューラー・ヒントン寒天プレート(MHAプレート)
- 50%のライズ馬の血液と20 mg/mL β-NAD(MH-F)のMHスープ
- E検定ペニシリン/ゲンタマイシン(または目的の抗生物質)(BioMerieux、マーシー・レトワール、フランス、スウェーデン)
- ペニシリン/ゲンタマイシン(または目的の抗生物質(粉末/溶液))
注:細菌の増殖に使用される特定の培地は、種によって異なる場合があります。
手順
1. エプシロメーター試験(E検定)
-
セットアップ
- 手袋とラボコートを着用する
- 70%エタノールを使用して殺菌してワークスペースを準備
- ミュラー・ヒントン寒天プレート(MHAプレート)を収集
-
マクファーランド濁度規格No.0.5の準備
- 塩化バリウム(BaCl2)の1%溶液を調調します。
100mL蒸留水に1グラムの無水バリウム塩化バリウム(BaCl2)を加えます。渦がよく。 - 硫酸の1%溶液を調出す(H2SO4):
蒸留水の99 mLに濃縮H2SO4の1 mLを加えます。渦がよく。 - マクファーランドの濁り標準番号0.5を準備します。
1%H2 SO4溶液の5mLで50 μL
- 塩化バリウム(BaCl2)の1%溶液を調調します。
結果
申請書と概要
参考文献
- Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): Discoverer of penicillin. Singapore Medical Journal. 56 (7):366-7. (2015)
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Frontiers in Microbiology. 1:134. (2010)
- Pankey GA, Ashcraft DS, Dornelles A. Comparison of 3 E-test (®) methods and time-kill assay for determination of antimicrobial synergy against carbapenemase-producing Klebsiella species. Diagnostic Microbiology and Infectious Disease. 77 (3):220-6. (2013)
- EUCAST: European Committee On Antimicrobial Susceptibility Testing (www.eucast.org).
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 3 (2):163-75. (2008)
- Doern CD, When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. Journal of Clinical Microbiology. 52 (12):4124-28. (2014)
- Worthington RJ, Melander C. Combination approaches to combat multi-drug resistant bacteria. Trends in Biotechnology. 31 (3):177-84. (2013)
タグ
スキップ先...
このコレクションのビデオ:

Now Playing
抗生物質感受性試験:2つの抗生物質のMIC値を決定し、抗生物質の相乗効果を評価するエプシロメーター試験
Microbiology
93.8K 閲覧数

ウィノグラツキーカラムの作成:堆積物サンプル中の微生物種を濃縮する方法
Microbiology
129.4K 閲覧数

シリアル希釈とめっき:微生物列挙
Microbiology
316.2K 閲覧数

エンリッチメント培養:選択的および差動媒体における好気性微生物と嫌気性微生物の培養
Microbiology
132.1K 閲覧数

純粋な培養物と縞めっき:混合サンプルからの単一細菌コロニーの単一の分離
Microbiology
166.2K 閲覧数

16S rRNAシーケンシング:細菌種を同定するPCRベースの技術
Microbiology
189.0K 閲覧数

成長曲線:コロニー形成単位と光学密度測定を用いて成長曲線を生成する
Microbiology
296.1K 閲覧数

顕微鏡検査と染色:グラム、カプセル、内胞染色
Microbiology
363.4K 閲覧数

プラークアッセイ:プラーク形成単位(PFU)としてウイルス定数を決定する方法
Microbiology
186.2K 閲覧数

適応塩化カルシウム手順を用いて大腸菌細胞の形質転換
Microbiology
86.8K 閲覧数

結合:アンピシリン耐性をドナーからレシピエント大腸菌に移す方法
Microbiology
38.2K 閲覧数

ファージトランスダクション:アンピシリン耐性をドナーからレシピエント大腸菌に伝達する方法
Microbiology
29.1K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved