Pruebas de susceptibilidad a los antibióticos: Pruebas con epsilometro para determinar los valores de la CMI de dos antibióticos y evaluar la sinergismos
Fuente: Anna Bl'ckberg1, Rolf Lood1
1 Departamento de Ciencias Clínicas Lund, División de Medicina de Infecciones, Centro Biomédico, Universidad de Lund, 221 00 Lund Suecia
El conocimiento de las interacciones entre antibióticos y bacterias es importante para entender cómo los microbios evolucionan la resistencia a los antibióticos. En 1928, Alexander Fleming descubrió la penicilina, un antibiótico que ejerce su función antibacteriana interfiriendo con la regeneración de la pared celular (1). Otros antibióticos con diversos mecanismos de acción han sido descubiertos posteriormente, incluyendo medicamentos que inhiben la replicación del ADN y la traducción de proteínas en bacterias; sin embargo, no se han desarrollado nuevos antibióticos en los últimos años. La resistencia a los antibióticos actuales ha ido en aumento, lo que ha dado lugar a enfermedades infecciosas graves que no pueden tratarse eficazmente (2). Aquí, describimos varios métodos para evaluar la resistencia a los antibióticos en poblaciones bacterianas. Cada uno de estos métodos funciona, independientemente del mecanismo de acción de los antibióticos utilizados, porque la muerte bacteriana es el resultado medido. La resistencia a los antibióticos no sólo se disemina rápidamente específicamente a través de los entornos hospitalarios, sino también en toda la sociedad. Con el fin de investigar estos medios de resistencia, se han desarrollado diferentes métodos, incluyendo la prueba del epsilometro (prueba E) y la prueba de dilución del caldo (3).
La prueba E es un método bien establecido y es una herramienta rentable que cuantifica los datos de la concentración mínima de inhibidores (MIC), la concentración más baja de un antimicrobiano que inhibe el crecimiento visible de un microorganismo. Dependiendo de la cepa bacteriana y de los antibióticos utilizados, el valor de la MIC puede variar entre sub g/ml y >1000 g/ml (4). La prueba E se realiza utilizando una tira de plástico que contiene un degradado antibiótico predefinido, que se imprime con la escala de lectura MIC en g/ml. Esta tira se transfiere directamente en la matriz de agar cuando se aplica a la placa de agar inoculada. Después de la incubación, una zona de inhibición elíptica simétrica es visible a lo largo de la tira a medida que se evita el crecimiento bacteriano. MIC se define por el área de inhibición, que es el punto final donde la elipse interseca la tira. Otro método común para determinar el MIC es el método de dilución de microcalina. La dilución de microcalina incorpora diferentes concentraciones del agente antimicrobiano añadido a un medio de caldo que contiene bacterias inoculadas. Después de la incubación, MIC se define como la concentración más baja de antibióticos que impide el crecimiento visible (5). También es un método cuantitativo y se puede aplicar a varias bacterias. Las desventajas de este método incluyen la posibilidad de errores al preparar las concentraciones de los reactivos y el gran número de reactivos necesarios para el experimento. La medición de la resistencia a los antibióticos es imprescindible tanto desde una perspectiva clínica como de investigación, y estos métodos in vitro para investigar la resistencia se discuten y se muestran a continuación.
El perfil de resistencia de una bacteria específica se puede aplicar con el fin de optimizar el tratamiento antibiótico para determinar si un paciente se beneficiaría del tratamiento combinado frente a la terapia única. Para el uso de más de un antibiótico a la vez, es imperativo conocer sus interacciones entre sí y si tienen un efecto aditivo, sinérgico o antagónico. Un efecto aditivo se puede ver cuando el efecto articular de los antibióticos es igual a la potencia de los antibióticos individuales dados a una dosis igual. La sinergia entre los antibióticos, por otro lado, está presente cuando el efecto articular de los antibióticos es más potente que si el medicamento se administrara solo (6). La aplicación de combinaciones de tratamiento antimicrobiano se utiliza para evitar la aparición de resistencia a los antimicrobianos, por lo tanto, para mejorar el efecto del tratamiento antibiótico individual (7). El conocimiento del antagonismo también es tan importante para prevenir el uso innecesario de combinaciones antimicrobianas. La metodología de prueba electrónico ofrece formas sencillas y varias de determinar posibles sinergias y antagonismo entre diferentes agentes antimicrobianos. Con el fin de hacer frente a la proliferación de patógenos resistentes a los antibióticos, el conocimiento de los posibles mecanismos sinérgicos y antagónicos de ciertos antibióticos es importante dando lugar a la eficacia clínica y luchando contra la resistencia multifármaco.
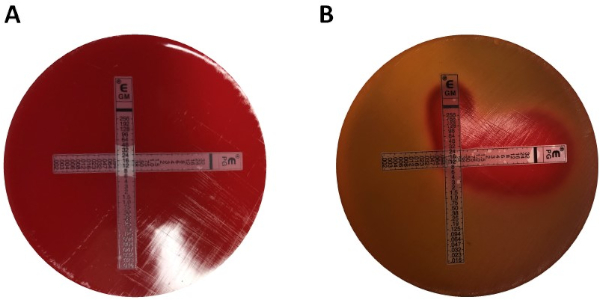
La determinación de la sinergia mediante pruebas E puede dividirse en dos enfoques generales: pruebas cruzadas y no cruzadas. Si bien ambas pruebas de sinergia se basan en el conocimiento previo de los valores individuales del MIC, los dos enfoques son ligeramente diferentes en metodología y enfoque conceptual. En una prueba de sinergia no cruzada, el primer antibiótico en el par a probar se coloca en una placa de agar inoculada con bacterias. Después de permitir que los antibióticos de la primera tira infundan la placa (por ejemplo, después de 1 hora), se retira la tira y se coloca una nueva tira que contiene el segundo antibiótico en el mismo lugar que la primera, asegurándose de colocar los dos valores individuales de MIC encima de cada ot Su. La zona de inhibición resultante se puede analizar como se describió anteriormente, y la sinergia calculada sobre la base de la Ecuación 1.
Ecuación 1 - Concentraciones inhibidoras fraccionarias (FIC)
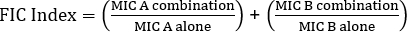
Valores >0.5 muestra sinergia.
Al recompensar al examinador con placas fáciles de analizar, el método es algo laborioso y lento debido al cambio de tiras, así como la necesidad de usar dos placas por experimento. En su lugar, a menudo se emplea una prueba cruzada. En lugar de añadir las dos tiras de prueba E diferentes posteriormente una encima de la otra (después de la eliminación de la primera), ambas se colocan simultáneamente pero en forma de una cruz (ángulo de 90o), con los dos valores MIC previamente determinados formando el ángulo de 90o. Con este enfoque sólo se necesita una placa por prueba de sinergia, así como menos trabajo, por lo que es una opción preferida a pesar de ser un poco más difícil de analizar. Los nuevos valores MIC en el enfoque combinado de antibióticos se pueden visualizar como las zonas de inhibición modificadas, después de lo cual la sinergia puede ser determinada por la Ecuación 1.
de 90o), con los dos valores MIC previamente determinados formando el ángulo de 90o. Con este enfoque sólo se necesita una placa por prueba de sinergia, así como menos trabajo, por lo que es una opción preferida a pesar de ser un poco más difícil de analizar. Los nuevos valores MIC en el enfoque combinado de antibióticos se pueden visualizar como las zonas de inhibición modificadas, después de lo cual la sinergia puede ser determinada por la Ecuación 1.
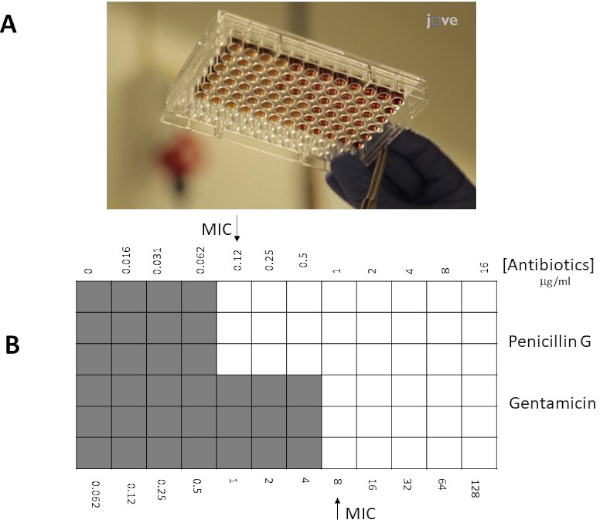
En lugar de utilizar un enfoque de placa de agar, un enfoque de microcaldo a menudo puede ser preferencial debido a su mayor flexibilidad (por ejemplo, la capacidad de elegir concentraciones específicas de antibióticos fuera de los límites de una tira de prueba E). Además, se sugiere que las pruebas de microcaldeo son más sensibles debido a su distribución uniforme de antibióticos en una solución líquida, no dependiendo de la disociación dentro de una fase sólida (placa de agar). Los pozos en una microplaca de 96 pocillos serán inoculados con un número determinado de bacterias (106 cfu/ml: la concentración bacteriana se puede estimar mediante mediciones OD600 nm, estándares de turbidez o por muestras de chapado de propagación de diluciones seriales bacterianas 10x), y antibióticos en diferentes diluciones se añadirán a los pozos. Del mismo modo, a las tiras de prueba E MIC se determina como la intersección (pozo/ punto) con la concentración más baja de antibióticos que inhiben el crecimiento visible de bacterias.
Objetivo experimental
- El siguiente proyecto describe estrategias para determinar los valores MIC de la penicilina G y la gentamicina del grupo G de Streptococcus mediante dos métodos diferentes, la prueba electrónica y la microdilución. Para la prueba E, las placas de agar Mueller-Hinton inoculadas con Streptococcus grupo G se utilizaron en combinación con tiras de gradiente de penicilina G y/o gentamicina; mientras que el caldo MH con 50% de sangre de caballo lysed y 20 mg/ml-NAD se utilizaron con antibióticos solubles junto con Streptococcus grupo G en un enfoque de microcaldo.
Materiales
- Colonias bacterianas en una placa de agar en sangre, almacenadas <7 días en 4oC
- Placas de agar de sangre
- 0.5 Estándar McFarland
- 1% BacI2
- 1% H2SO4
- Tubo salino (2 mL)
- Aplicador con punta de algodón
- Placas de agar Mueller-Hinton (placas MHA)
- Caldo MH con 50% de sangre de caballo lysed y 20 mg/mL-NAD (MH-F)
- E-test penicilina/gentamicina (o antibióticos de interés) (BioMerieux, Marcy l'Etoile, Francia, Suecia)
- Antibióticos penicilina/gentamicina (o antibióticos de interés (polvo/solución))
Nota: Los medios específicos utilizados para el crecimiento bacteriano pueden variar para diferentes especies.
1. Pruebas de epsiómetro (pruebas E)
-
Configuración
- Use guantes y un abrigo de laboratorio
- Preparar el espacio de trabajo esterilizando con 70% de etanol
- Recoger placas de agar Mueller-Hinton (placas MHA)
-
Preparación de un estándar de turbidez de McFarland No. 0.5
- Preparar una solución al 1% de cloruro de bario (BaCl2):
Añadir 1 gramo de cloruro de bario anhidro (BaCl2) e
- Preparar una solución al 1% de cloruro de bario (BaCl2):
Valores MIC en la prueba E
Los valores individuales de MIC se identificaron en la Figura 1 como 0,094 g/ml para la penicilina G y 8 g/ml para la gentamicina. Para las pruebas de sinergia, ambas demostraron un valor MIC para la penicilina G de 0,064 g/ml(Figuras 2, 3),mientras que la gentamicina tenía un MIC de 4 g/ml para pruebas cruzadas y no cruzadas. Tenga en cuenta que puede producirse una ligera discrepancia entre las pruebas cruzadas y .
La resistencia a los antibióticos es un problema de salud mundial. Con el fin de determinar los mecanismos de resistencia de los microbios, los métodos de prueba de sinergia y antagonismo con diferentes antibióticos es crucial. El método de prueba E es rápido, fácil de replicar y se puede utilizar para investigar cualquier potencial sinérgico de las terapias combinadas. El método de dilución del caldo también se puede evaluar para predecir la actividad bactericida. Con el fin de investigar los mecanismos de res...
- Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): Discoverer of penicillin. Singapore Medical Journal. 56 (7):366-7. (2015)
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Frontiers in Microbiology. 1:134. (2010)
- Pankey GA, Ashcraft DS, Dornelles A. Comparison of 3 E-test (®) methods and time-kill assay for determination of antimicrobial synergy against carbapenemase-producing Klebsiella species. Diagnostic Microbiology and Infectious Disease. 77 (3):220-6. (2013)
- EUCAST: European Committee On Antimicrobial Susceptibility Testing (www.eucast.org).
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 3 (2):163-75. (2008)
- Doern CD, When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. Journal of Clinical Microbiology. 52 (12):4124-28. (2014)
- Worthington RJ, Melander C. Combination approaches to combat multi-drug resistant bacteria. Trends in Biotechnology. 31 (3):177-84. (2013)
Saltar a...
Vídeos de esta colección:

Now Playing
Pruebas de susceptibilidad a los antibióticos: Pruebas con epsilometro para determinar los valores de la CMI de dos antibióticos y evaluar la sinergismos
Microbiology
93.3K Vistas

Creación de la columna de Winogradsky: Un método que sirve para enriquecer las especies microbianas en una muestra de sedimento
Microbiology
127.4K Vistas

Diluciones en serie y enchapado: enumeración microbiana
Microbiology
312.4K Vistas

Cultivos de enriquecimiento: Cultivo de bacterias aerobias y anaerobias en medios selectivos y diferenciales
Microbiology
131.5K Vistas

Cultivos puros y siembra por estrías: aislamiento de colonias bacterianas únicas de una muestra mixta
Microbiology
165.3K Vistas

Secuenciación del ARNr 16s: Una técnica basada en PCR para identificar especies bacterianas
Microbiology
187.2K Vistas

Curvas de crecimiento: Generación de curvas de crecimiento utilizando unidades formadoras de colonias y mediciones de densidad óptica
Microbiology
291.0K Vistas

Microscopía y tinción: Tinción de Gram, cápsula y endosporas
Microbiology
361.9K Vistas

Ensayo de placa: Un método para determinar los títulos virales como unidades formadoras de placas (UFP)
Microbiology
185.1K Vistas

Transformación de células E. coli mediante un procedimiento con cloruro de calcio
Microbiology
86.0K Vistas

Conjugación: Un método para transferir resistencia a ampicilina de un donante a una E. Coli receptora
Microbiology
37.9K Vistas

Transducción de fagos: Un método para transferir resistencia a ampicilina de un donante una E. coli receptora
Microbiology
28.8K Vistas
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados