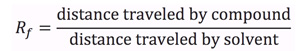Performing 1D Thin Layer Chromatography
概要
Source: Laboratory of Dr. Yuri Bolshan — University of Ontario Institute of Technology
Thin layer chromatography (TLC) is a chromatographic method used to separate mixtures of non-volatile compounds. A TLC plate consists of a thin layer of adsorbent material (the stationary phase) fixed to an appropriate solid support such as plastic, aluminum, or glass1. The sample(s) and reference compound(s) are dissolved in an appropriate solvent and applied near the bottom edge of the TLC plate in small spots. The TLC plate is developed by immersing the bottom edge in the developing solvent consisting of an appropriate mobile phase. Capillary action allows the mobile phase to move up the adsorbent layer. As the solvent moves up the TLC plate, it carries with it the components of each spot and separates them based on their physical interactions with the mobile and stationary phases.
手順
1. TLC Plates
- Common adsorbents for TLC are silica gel, alumina, and cellulose. TLC plates are commercially available with a variety of properties. Choose a TLC plate and cut it down to an appropriate size (approximately 5 cm x 5 cm is sufficient for most applications). For glass-backed TLC plates, score the glass using a ruler and a glass cutter, then carefully break along the line.
2. Spotting
- Dissolve the sample in a suitable solvent to make an approximately 1
結果
An example of a typical TLC plate is shown in Figure 1. An unknown compound 'A' may be compared to known standards 'B' through 'E'. Determination of the Rf value for each component is achieved by spotting of each respective compound, developing the TLC plate, and visualization. The Rf of unknown compound 'A' is calculated by measuring the spot height (y) and dividing by the solvent height (z). Comparing this
申請書と概要
TLC has a number of practical applications in the laboratory. TLC may be used to identify unknown compounds and unknown components of mixtures via comparison with standards. TLC is commonly used to monitor the course of a chemical reaction, and to assess the purity of the product through the comparison of relative amounts of reactants, products, and by-products on successive chromatograms over time. TLC can also be used to determine if a substance purified by other methods (such as recrystallization or distillation) stil
参考文献
- Lehman, J. W. The student's lab companion: laboratory techniques for organic chemistry: standard scale and microscale. Pearson College Div, (2008).
- Pavia, D. L., Lampman, G. M., Kriz, G. S., & Engel, R. G. Microscale and Macroscale Techniques. Thomson Wadsworth, (2006).
- Pannkuk, E. L., Risch, T. S., Savary, B. J. Profiling the Triacylglyceride Contents in Bat Integumentary Lipids by Preparative Thin Layer Chromatography and MALDI-TOF Mass Spectrometry. J. Vis. Exp. (79), e50757, (2013).
- Kagan, I. A., Flythe, M. D. Thin-layer Chromatographic (TLC) Separations and Bioassays of Plant Extracts to Identify Antimicrobial Compounds. J. Vis. Exp. (85), e51411, (2014).
タグ
スキップ先...
このコレクションのビデオ:

Now Playing
Performing 1D Thin Layer Chromatography
Organic Chemistry
289.7K 閲覧数

触媒入門
Organic Chemistry
34.5K 閲覧数

温水の化学反応のための還流システムの組み立て
Organic Chemistry
167.5K 閲覧数

室温以下の反応を実施
Organic Chemistry
70.6K 閲覧数

Schlenk ライン溶剤伝
Organic Chemistry
41.6K 閲覧数

凍害ポンプ サイクリングで液体の脱気
Organic Chemistry
56.1K 閲覧数

無水試薬と機器の準備
Organic Chemistry
79.4K 閲覧数

再結晶により物質を浄化
Organic Chemistry
708.6K 閲覧数

沈殿物によって混合物の分離
Organic Chemistry
157.8K 閲覧数

固液抽出
Organic Chemistry
237.8K 閲覧数

溶媒を除去する回転蒸発
Organic Chemistry
212.9K 閲覧数

分別蒸留
Organic Chemistry
334.4K 閲覧数

X 線回折用結晶を成長
Organic Chemistry
32.4K 閲覧数

カラム ・ クロマトグラフィ
Organic Chemistry
360.1K 閲覧数

核磁気共鳴 (NMR) 分光法
Organic Chemistry
247.8K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved