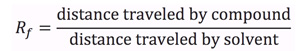Performing 1D Thin Layer Chromatography
Overview
Source: Laboratory of Dr. Yuri Bolshan — University of Ontario Institute of Technology
Thin layer chromatography (TLC) is a chromatographic method used to separate mixtures of non-volatile compounds. A TLC plate consists of a thin layer of adsorbent material (the stationary phase) fixed to an appropriate solid support such as plastic, aluminum, or glass1. The sample(s) and reference compound(s) are dissolved in an appropriate solvent and applied near the bottom edge of the TLC plate in small spots. The TLC plate is developed by immersing the bottom edge in the developing solvent consisting of an appropriate mobile phase. Capillary action allows the mobile phase to move up the adsorbent layer. As the solvent moves up the TLC plate, it carries with it the components of each spot and separates them based on their physical interactions with the mobile and stationary phases.
Procedure
1. TLC Plates
- Common adsorbents for TLC are silica gel, alumina, and cellulose. TLC plates are commercially available with a variety of properties. Choose a TLC plate and cut it down to an appropriate size (approximately 5 cm x 5 cm is sufficient for most applications). For glass-backed TLC plates, score the glass using a ruler and a glass cutter, then carefully break along the line.
2. Spotting
- Dissolve the sample in a suitable solvent to make an approximately 1
Results
An example of a typical TLC plate is shown in Figure 1. An unknown compound 'A' may be compared to known standards 'B' through 'E'. Determination of the Rf value for each component is achieved by spotting of each respective compound, developing the TLC plate, and visualization. The Rf of unknown compound 'A' is calculated by measuring the spot height (y) and dividing by the solvent height (z). Comparing this
Application and Summary
TLC has a number of practical applications in the laboratory. TLC may be used to identify unknown compounds and unknown components of mixtures via comparison with standards. TLC is commonly used to monitor the course of a chemical reaction, and to assess the purity of the product through the comparison of relative amounts of reactants, products, and by-products on successive chromatograms over time. TLC can also be used to determine if a substance purified by other methods (such as recrystallization or distillation) stil
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
Performing 1D Thin Layer Chromatography
Organic Chemistry
290.1K Views

촉매 소개
Organic Chemistry
34.6K Views

가열된 화학 반응을 위한 역류 시스템의 조립
Organic Chemistry
168.3K Views

실온 이하의 반응 수행
Organic Chemistry
70.7K Views

솔벤트의 슐렌크 라인 전송
Organic Chemistry
41.7K Views

동결 펌프 해동 사이클링으로 액체 를 탈기
Organic Chemistry
56.3K Views

무수성 시약 및 장비 준비
Organic Chemistry
79.4K Views

재결정화로 화합물 정화
Organic Chemistry
710.1K Views

침전을 통한 혼합물의 분리
Organic Chemistry
158.0K Views

고체 액체 추출
Organic Chemistry
238.1K Views

용매제거를 위한 로타리 증발
Organic Chemistry
213.0K Views

분수 증류
Organic Chemistry
334.8K Views

X선 회절 분석을 위한 커지는 결정
Organic Chemistry
32.9K Views

열 크로마토그래피
Organic Chemistry
360.9K Views

핵 자기 공명 (NMR) 분광기
Organic Chemistry
248.9K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유