JoVE 비디오를 활용하시려면 도서관을 통한 기관 구독이 필요합니다. 전체 비디오를 보시려면 로그인하거나 무료 트라이얼을 시작하세요.
Method Article
Application of High-speed Super-resolution SPEED Microscopy in Live Primary Cilium
요약
Recently we mapped the three-dimensional (3D) spatial locations of transport routes for various proteins translocating inside primary cilia in live cells. Here this paper details the experimental setup, the process of biological samples and the data analyses for the 3D super-resolution fluorescence imaging approach newly applied in live primary cilia.
초록
The primary cilium is a microtubule-based protrusion on the surface of many eukaryotic cells and contains a unique complement of proteins that function critically in cell motility and signaling. Since cilia are incapable of synthesizing their own protein, nearly 200 unique ciliary proteins need to be trafficked between the cytosol and primary cilia. However, it is still a technical challenge to map three-dimensional (3D) locations of transport pathways for these proteins in live primary cilia due to the limitations of currently existing techniques. To conquer the challenge, recently we have developed and employed a high-speed virtual 3D super-resolution microscopy, termed single-point edge-excitation sub-diffraction (SPEED) microscopy, to determine the 3D spatial location of transport pathways for both cytosolic and membrane proteins in primary cilia of live cells. In this article, we will demonstrate the detailed setup of SPEED microscopy, the preparation of cells expressing fluorescence-protein-labeled ciliary proteins, the real-time single-molecule tracking of individual proteins in live cilium and the achievement of 3D spatial probability density maps of transport routes for ciliary proteins.
서문
Since stated by Ernst Abbe in 1873, the resolution of conventional light microscopy has been believed to be limited to approximately 200 nm due to light diffraction from the objective1,2. Currently, super-resolution light microscopy techniques break this limitation and allow the capture of dynamic images with sub-diffraction (< 200 nm) resolution. The techniques generally fall into two broad categories: stimulated emission depletion (STED) microscopy based approaches, which generate sub-diffraction illumination volume due to nonlinear optical response of fluorophores in samples3; and photoactivated light microscopy (PALM) and stochastic optical reconstruction microscopy (STORM)-based super-resolution techniques, which utilize mathematical functions to localize the centroids of fluorophores and then reconstitute these centroids to form super-resolution images4,5. Currently, due to the relatively uncomplicated optical setup, PALM and STORM are extensively employed by only activating a small subset of fluorophores in each frame of a long video of a biological preparation. This allows for the more accurate localization by 2D Gaussian fitting of the fluorescent spot, termed the point spread function (PSF), of fluorescently-labeled proteins in each frame of the video. The 2D location of each fluorescently-labeled molecule can then be superimposed on a single imaging plane to produce a super-resolution image of the biological preparation1,2. While these single-molecule localization, super-resolution approaches to microscopy certainly revolutionized how imaging of biological samples was performed, there are still challenges to be overcome. For example, STORM and PALM can achieve their best spatial resolutions after fixation of biological samples and thus present a static representation of the fluorescently-labeled proteins, which is a similar limitation of electron microscopy. Additionally, to achieve high spatial resolution for each fluorescently-labeled protein in live cells, samples must be imaged at very long framerates which are unable to capture protein dynamics. Therefore, it is necessary to overcome these main technical hurdles.
To obtain a high spatiotemporal resolution that is well-suited for detecting fast-moving proteins or RNAs in live cells, we have developed super-resolution SPEED microscopy in our laboratory (Figure 1)6,7,8. Several major technical advances in SPEED microscopy have previously enabled us to successfully track nucleocytoplasmic transport of small molecules, proteins, mRNA and virus through native nuclear pore complexes (NPCs)6,7,8. Briefly, the following features of SPEED microscopy will be used to track fast-moving macromolecules through sub-micrometer rotationally symmetrical structures in live cells, such as NPCs and primary cilia: (1) An inclined or a vertical illumination PSF enables the excitation of single molecules within a small diffraction-limit volume in the focal plane (Figure 1); (2) The inclined PSF can greatly avoid out-of-focus fluorescence and thus improve the signal-to-noise ratio. (3) The optical density of 100-500 kW/ cm2 in the illumination PSF allows thousands of photons to be collected from single fluorophores with fast detection speeds (> 500 Hz). (4) The fast detection speed also greatly reduces the single-molecule spatial localization error (< 10 nm) in determining the spatial trajectories of moving fluorescent molecules in live cells, because molecular diffusion is one of major factors causing imperfections of single-molecule localization for moving molecules. (5) Well-established 2D to 3D transformation algorithms enable us to provide 3D spatial probability density maps of transport routes for molecules in the NPC or the primary cilium. It is noteworthy that our conversion process between the Cartesian and the cylindrical coordination system is used to generate a 3D spatial probability density map rather than 3D single-molecule tracking (Figure 2). Previously, electron microscopy data have revealed that the NPC9,10 and the primary cilium11 both have a rotationally symmetrical structure. In principle, randomly diffusing molecules moving through the NPC or primary cilium should also have rotationally symmetrical distributions. As shown in Figure 2, a high number of randomly diffusing molecules inside the cylinder would generate rotationally symmetrical distributions at the cross-section view as that in the NPC, further resulting in an approximately uniform spatial distribution within each very small sub-region between two neighboring rings (Figure 2E). This uniform distribution leads that the spatial distribution along θ dimension in the cylindrical system is constant. Then the 3D coordinates (R, X, θ) can be simplified to be the 2D coordinates (R, X, constant). Actually, our conversion process between the Cartesian and the cylindrical systems is from 2D (X, Y) to 2D (R, X, constant). The constant θ, refers to the spatial density p in Figure 2E, is calculated by using the equation A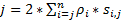 .
.
Ultimately, single-molecule tracking has broad application in biological research, thus, it is natural that a plethora of techniques will be developed to fill specific biological niches12,13,14. Such is the case with SPEED microscopy. Previously, when coupled with a 3D transformation algorithm, this technique was developed to resolve 3D transport routes of transiting molecules through the NPCs, a sub-diffraction-sized and rotationally symmetric biological structure6. In this paper, primary cilia are shown to be excellent model organelles as well. Primary cilia are cylindrical, antenna-like organelles (~125 nm radius) that project from the surface of most mammalian cells15,16,17. They are responsible for receiving external signals and transmitting an intracellular response typically associated with growth and metabolism15,16. Therefore, flux of structural proteins, recycling of transmembrane receptors, and transmission of intracellular messengers are vital responsibilities of primary cilia. At the juncture between the primary cilia and the cell body is a critical selectivity barrier, called the transition zone or TZ, through which all this protein transport must occur11,18,19,20. In addition to the gating function of the TZ, at least two transport processes, intraflagellar transport and passive diffusion, are thought to be responsible for the movement of protein through this region16,21,22. From a human health standpoint, the loss of primary cilia and subsequent deregulation of downstream signaling is characteristic of many cancers. In addition, many genetic diseases, such as Bardet-Biedl syndrome and polycystic kidney disease, are associated with defective protein transport23. Both the sub-diffraction limit size and the complex process of selective protein transport through the TZ make the primary cilia a prime target for this technique. In this methods paper, we will demonstrate the tracking of a ciliary transmembrane protein, somatostatin receptor 3 (SSTR3)24, labeled externally with Alexa Fluor 647 and a component of IFT, IFT2025, labeled with a fused GFP molecule.
프로토콜
1. NIH-3T3 cell preparation for SPEED microscopy from stock
- 1.5 weeks in advance of the experiment, recover a fresh culture of NIH-3T3 cells from a frozen stock by thawing at 37 °C and transferring the cells to a 25 cm2 cell culture flask with 3 mL of Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 110 mg/mL sodium pyruvate, 2 mM glutamine, 10% fetal bovine serum, and 1% penicillin/streptomycin.
- Incubate the cells at 37 °C in a 5% CO2 incubator.
- Split cells at 80% confluency, about every two days, at least three times before experimental day to ensure homogeneity of cell cycle. Trypsinize cells with 0.25% trypsin for 2 min at 37 °C, aspirate trypsin and replace it with 2 mL of medium. Pipette the medium repeatedly to break up cell clusters, remove the desired number of cells and bring total volume of media back up to 3 mL.
NOTE: NIH-3T3 were previously genetically engineered to express NPHP-4, a protein that localizes to the TZ26, fused at the C terminus to mCherry. mCherry is a fluorophore which can be excited with 561 nm illumination to quantitatively localize the TZ selectivity barrier and orient the primary cilia. - Two days before the experiment, plate the cells into a 35 mm glass bottom dish at 60-70% confluency with 1.5 mL of the same medium as step 1.1 and return the cells to the incubator.
- One day before the experiment, chemically transfect the cells with the desired plasmid. Mix 500 - 1000 ng of the desired plasmid (see the Note below) in a 1:2.5 ratio with transfection reagent in 0.25 mL of reduced serum media without antibiotics for 30 min. Aspirate media from the 35 mm glass bottom dish and replace it with the 0.25 mL plasmid/transfection reagent mix plus an extra 1.25 mL of reduced serum media without antibiotics. Reduced serum media serves the purpose facilitating a successful transfection, inducing primary cilia growth, as well as keeping the cells alive long enough to perform experiment. Return cells to the incubator for the experiment on the following day.
NOTE: When performing single-molecule tracking of IFT20, a plasmid containing a genetically-modified IFT20 fused at its C terminus to GFP is used25. When performing single-molecule tracking of SSTR3, a plasmid containing a genetically-modified SSTR3 fused at its N terminus to an acceptor peptide (AP) domain and C terminus to GFP is used22. In addition to the SSTR3 construct, a plasmid containing the biotin ligase BirA must be co-expressed and the transfection media must be supplemented with 10 µM biotin. BirA then attaches biotin to the AP domain of newly synthesized AP-SSTR3-GFP molecules at the level of the ER. Alexa647 conjugated to three of the four biotin-binding sites on streptavidin, on average, may then be supplemented to the media prior to imaging to fluorescently label the AP-SSTR3-GFP molecules on the external surface of the cell22,27. GFP and AlexaFluor647 are used in this method; however, other fluorescent probes can be used if they have similarly high photo-stability and quantum yield. - If using the externally-labeled SSTR3 construct, remove the media from the glass bottom dish 1 h before experiment, wash the cell 5 times with 1 mL of phosphate-buffered saline (PBS), and add 1 mL of reduced serum media supplemented with 1 µM Alexa647 conjugated streptavidin.
- No more than 15 min before the experiment, remove media from the glass bottom dish and wash the transfected and labeled cells 5 times with 1 mL of PBS.
- Place 1 mL of imaging buffer (20 mM HEPES, 110 mM KOAc, 5 mM NaOAc, 2 mM MgOAc, 1 mM EGTA, pH 7.3) in the glass bottom dish.
NOTE: In the imaging buffer, cells are viable for no longer than 3 h. Therefore, only 2 h of experiments are performed on each dish.
2. SPEED microscopy
Note: The SPEED microscopy setup includes an inverted fluorescence microscope equipped with a 1.4-NA 100× oil-immersion apochromatic objective, a 35 mW 633 nm He-Ne laser, 50 mW solid state 488-nm and 561-nm lasers, an on-chip multiplication gain charge-coupled-device camera and a microscope software package for data acquisition and processing (Figure 1). For individual channel imaging, GFP, mCherry, and Alexa647 are excited by 488 nm, 561 nm, or 633 nm lasers, respectively. For single molecule tracking, single point illumination is used to track individual fluorescently-labeled molecules. For epifluorescence imaging, a concave lens is placed in the laser illumination path to expand the beam into a uniform field of illumination. The fluorescence emissions are collected by the same objective, filtered by a dichroic filter (405/488/561/635) and an emission filter (405/488/561/635), and imaged with the above CCD camera operating at 500 Hz for single molecule tracking or 2 Hz for epifluorescence imaging.
- Affix the glass bottom plate to the stage of the microscope and locate a cell that is properly expressing the desired constructs. Once a suitable cell has been found, align the NPHP4-mCherry spot at the base of the primary cilia with the location on the imaging plane that corresponds to the laser's single point illumination.
- Capture an epifluorescence image of NPHP4-mCherry and either IFT20-GFP or AP-SSTR3-GFP using the "Snap" function in the "Camera" tab of the "Focus Controls" window if using the digital microscopy software package (see Table of Materials).
NOTE: These images will act as a reference for the subsequent single molecule locations. - Once the reference images are obtained, locally reduce the concentration of labeled single molecules. Photo-bleach the TZ with 1 mW laser illumination for 20 s or until the fluorescence intensity is close to that of background fluorescence.
NOTE: When the precise concentration can be controlled, 0.1-1 nM labeled single molecules are used. - To prepare for single molecule tracking, reduce the laser illumination power to ~0.15 mW for single molecules labeled with GFP or ~0.5 mW for molecules labeled with Alexa647.
- As soon as the laser power and imaging parameters are set, maximum gain and intensification and 2 ms frame rate, for single molecule imaging, engage the appropriate illumination laser and record non-photobleached, labeled single molecules as they are transported through the photobleached region of the TZ by clicking the "Stream" button in the "Camera" tab of the "Focus Controls" window.
NOTE: No more than 2 min of video should be captured to minimize the effects of ciliary drift to a negligible level. - After capturing the single molecule video, process the videos using a 2D Gaussian fitting algorithm, such as Glimpse by the Gelles Lab, which precisely localizes the centroid of each single molecule's excitation PSF in an encompassing area of interest (AOI).
- Select all single molecule locations with precision <10 nm and correct the center of cilia based on distribution of single molecule locations fitted with a 2D Gaussian function.
NOTE: By using 2D to 3D transformation algorithm, the 3D transport routes of IFT20-GFP and AP-SSTR3 routes are clearly shown on ciliary axonemal or ciliary membrane, respectively.
3. 2D to 3D Transformation
- Once several thousand localizations for transiting molecules (signal to noise ratio > 11) in the cilium are collected, select the long axis of the cilium as the X-dimension. Make a Y dimension histogram of the locations and obtain the bin sums in 10 nm increments.
NOTE: The 2D to 3D transformation may be evaluated by hand or any software or programming language. The authors have successfully implemented the transformation in both Matlab and Python 2.7.
결과
This section demonstrates the data obtained from performing SPEED microscopy at the TZ of primary cilia to study the transport route of SSTR3 connected by a ~15 nm external linker to Alexa647 (Figure 3A). It serves the dual purpose of verifying the 3D transformation algorithm. Alexa647 should only label the external surface of the primary cilium and therefore, the 3D transport route should reveal a high-density transport route at that locatio...
토론
This protocol describes the application of SPEED microscopy to the primary cilium, a cellular signaling organelle that is highly reliant on efficient protein transport. SPEED microscopy can provide high resolution (< 10 nm) locations for fluorescently-labeled molecules as they pass through the single point illumination centered on the TZ. Previously it has been applied to study the protein trafficking through the NPC6,7,8. H...
공개
The authors declare no conflicts of interest.
감사의 말
We thank Dr. Kristen Verhey (University of Michigan, Ann Arbor) and Dr. Gregory Pazour (University of Massachusetts Medical School) for providing some plasmids. The project was supported by grants from the National Institutes of Health (NIH GM097037, GM116204 and GM122552 to W.Y.).
자료
| Name | Company | Catalog Number | Comments |
| 25 cm2 tissue culture dish | Corning | VV-01936-00 | |
| Penicillin/streptomycin | ThermoFisher | 15140122 | |
| Fetal bovine serum | ThermoFisher | 10438018 | |
| DMEM | ThermoFisher | 10566-016 | |
| OPTIMEM | ThermoFisher | 31985062 | |
| Trypsin | ThermoFisher | 25300054 | |
| Phosphate buffered saline | Sigma-Aldrich | P3813-1PAK | |
| Transit LT1 | Mirus | MIR 2300 | |
| 35 mm glass bottom dish | MatTek | P35GCOL-0-14-C | |
| AlexaFluor 647-conjugated streptavidin | ThermoFisher | S21374 | |
| Biotin | Sigma-Aldrich | B4501-100MG | |
| 633 nm He-Ne laser | Melles Griot | 25-LHP-928-249 | |
| 561 nm solid state laser | Coherent | OBIS 561-50 LS | |
| 488 nm solid state laser | Coherent | 1185053 | |
| Inverted fluorescence microscope | Olympus | IX81 | |
| 1.4-NA 100× oil-immersion apochromatic objective | Olympus | UPLSAPO 100× | |
| On-chip multiplication gain charge-coupled-device camera | Roper Scientific | Cascade 128+ | |
| Dichroic filter | Semrock | Di01- R405/488/561/635-25x36 | |
| Emission filter | Semrock | NF01-405/488/561/635-25X5.0 | |
| Slidebook 6.0 | Intelligent Imaging Innovations | digital microscopy software |
참고문헌
- Huang, B., Bates, M., Zhuang, X. Super-resolution fluorescence microscopy. Annu Rev Biochem. 78, 993-1016 (2009).
- Leung, B. O., Chou, K. C. Review of super-resolution fluorescence microscopy for biology. Appl Spectrosc. 65, 967-980 (2011).
- Willig, K. I., Rizzoli, S. O., Westphal, V., Jahn, R., Hell, S. W. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 440, 935-939 (2006).
- Betzig, E., et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 313, 1642-1645 (2006).
- Rust, M. J., Bates, M., Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Meth. 3, 793-796 (2006).
- Ma, J., Yang, W. Three-dimensional distribution of transient interactions in the nuclear pore complex obtained from single-molecule snapshots. Proc Natl Acad Sci USA. 107, 7305-7310 (2010).
- Ma, J., Goryaynov, A., Sarma, A., Yang, W. Self-regulated viscous channel in the nuclear pore complex. Proc Natl Acad Sci USA. 109, 7326-7331 (2012).
- Ma, J., et al. High-resolution three-dimensional mapping of mRNA export through the nuclear pore. Nat Comm. 4, (2013).
- Akey, C. W., Radermacher, M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 122, 1-19 (1993).
- Akey, C. W. Interactions and structure of the nuclear pore complex revealed by cryo-electron microscopy. J Cell Biol. 109, 955-970 (1989).
- Czarnecki, P. G., Shah, J. V. The ciliary transition zone: from morphology and molecules to medicine. Trends Cell Biol. 22, 201-210 (2012).
- Elf, J., Li, G. -. W., Xie, X. S. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 316, 1191-1194 (2007).
- Anzalone, A., Annibale, P., Gratton, E. 3D orbital tracking in a modified two-photon microscope: an application to the tracking of intracellular vesicles. J Vis Exp. , (2014).
- Ritter, J. G., Veith, R., Veenendaal, A., Siebrasse, J. P., Kubitscheck, U. Light sheet microscopy for single molecule tracking in living tissue. PloS one. 5, 11639 (2010).
- Marshall, W. F., Nonaka, S. Cilia: tuning in to the cell's antenna. Curr Biol. 16, 604-614 (2006).
- Scholey, J. M., Anderson, K. V. Intraflagellar transport and cilium-based signaling. Cell. 125, 439-442 (2006).
- Yang, T. T., et al. Superresolution pattern recognition reveals the architectural map of the ciliary transition zone. Sci Rep. 5, 14096 (2015).
- Craige, B., et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 190, 927-940 (2010).
- Kee, H. L., et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol. 14, 431-437 (2012).
- Najafi, M., Maza, N. A., Calvert, P. D. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci USA. 109, 203-208 (2012).
- Nachury, M. V., Seeley, E. S., Jin, H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier. Annu Rev Cell Dev Biol. 26, 59-87 (2010).
- Ye, F., et al. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife. 2, 00654 (2013).
- Ross, A. J., et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genetics. 37, 1135-1140 (2005).
- Handel, M., et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 89, 909-926 (1999).
- Follit, J. A., Tuft, R. A., Fogarty, K. E., Pazour, G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 17, 3781-3792 (2006).
- Awata, J., et al. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J Cell Sci. 127, 4714-4727 (2014).
- Howarth, M., Ting, A. Y. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc. 3, 534-545 (2008).
- Huang, B., Wang, W., Bates, M., Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science. 319, 810-813 (2008).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유