JoVE 비디오를 활용하시려면 도서관을 통한 기관 구독이 필요합니다. 전체 비디오를 보시려면 로그인하거나 무료 트라이얼을 시작하세요.
Method Article
머드 크랩의 난소 성숙을 증가시키는 눈썹 절제
Erratum Notice
요약
마취된 암컷 게에 대해 두 가지 안구 절제 프로토콜(즉, 소작 및 수술 접근법)을 수행했습니다. 진흙 게의 눈썹 절제는 생존율을 감소시키지 않으면서 난소의 숙성을 앞당겼습니다.
초록
머드 크랩(Scylla spp.)은 인도-서태평양 지역에서 볼 수 있는 상업적으로 중요한 갑각류 종입니다. 배양 중에 난소 성숙의 유도는 성숙한 머드 크랩에 대한 소비자 수요를 충족시키고 종자 생산을 촉진하는 데 중요합니다. 눈썹 절제술은 진흙 게의 난소 성숙을 향상시키는 효과적인 도구입니다. 그러나 진흙 게의 눈웍 절제에 대한 표준 프로토콜은 없습니다. 이 연구에서는 소작(마취된 게의 눈줄기를 제거하기 위해 뜨거운 금속을 사용하는 것)과 수술(수술용 가위를 사용하여 눈줄기를 제거하는 것)의 두 가지 안자 절제 기술에 대해 설명합니다. 안자 절제 전에 성적으로 성숙한 여성(CW > 86mm)을 바닷물과 함께 얼음 주머니(-20°C)를 사용하여 마취했습니다. 수온이 4°C에 도달했을 때, 얼음 주머니를 물에서 제거하였다. 흐르는 바닷물(주위온도: 28°C)은 안쌥절제 직후 마취 회복을 위해 사용하였다. 사망률은 안구 절제 과정 중 또는 이후에 발생하지 않았습니다. 여기에 제시된 안대 절제 프로토콜은 진흙 게의 난소 성숙을 가속화했습니다.
서문
Scylla 속에 속하는 4 종의 머드 크랩 종은 모두 양식업에서 상업적으로 중요한 갑각류 종입니다 1,2. 진흙 게를 포함한 갑각류의 성장과 미숙 (아성체 또는 사춘기) 단계에서 성적으로 성숙한 (성체) 단계로의 전환은 오래되고 작은 외골격을 주기적으로 흘리는 탈피 과정을 통해 발생합니다. 갑각 너비(CW), chelipeds 및 복부 플랩 형태는 Scyll a spp.의 성적 성숙도를 결정하는 데 널리 사용됩니다. 3,4,5. 털갈이 과정은 다양한 호르몬의 작용에 의해 조절되며 엄청난 양의 에너지가 필요합니다6. 정상적인 털갈이 과정 외에도, 자발적으로 또는 외부 요인에 의해 유발 된 팔다리의 손실은 생존율에 영향을 미치지 않으면 서 게의 털갈이를 촉진합니다 7,8,9. 따라서 사지 자율절제술은 연질 껍질 머드 크랩 양식 산업 7,9에서 털갈이 유도에 일반적으로 사용됩니다.
일방적 또는 양측 눈썹 절제술은 생식선 성숙 및 종자 생산을 위해 민물 새우와 해양 새우에서 주로 인기가 있습니다10,11,12,13. 갑각류의 일반적인 안자 절제 기술은 다음을 포함한다: (i) 끈을 이용한 안자 기저부에서의 결찰14,15; (ii) 고온 집게 또는 전기 소작 장치(16)를 사용한 안자의 소작; (iii) 열린 상처를 남기기 위해 눈썹을 제거하거나 직접 꼬집는 단계(12); 및 (iv) 면도칼로 눈의 말단부를 절단한 후 절개를 통해 안자 내용물을 제거하는 단계(17). 눈썹 X 기관은 갑각류 고혈당 호르몬 (CHH), 털갈이 억제 호르몬 (MIH) 및 vitellogenes-inhibiting hormones (VIH)를 조절하기 때문에 갑각류의 중요한 내분비 기관입니다 6,18,19,20,21,22. 눈줄기 X-기관(또는 부비동 샘 복합체)은 신경펩티드 호르몬 계열에 속하는 성선 형성 억제 호르몬(VIH)이라고도 하는 생식선 억제 호르몬(GIH)을 합성하고 방출한다6. 편측성 또는 양측성 안켠 절제술은 GIH 합성을 감소시켜 자극 호르몬(즉, 생식선 자극 호르몬, GSH)의 우세와 갑각류의 난소 성숙 과정을 가속화한다23,24,25,26. 눈 줄기 절제 후 GIH의 영향이 없는 암컷 갑각류는 난소 발달에 에너지를 쏟는다27. 일방적인 안대 절제술은 갑각류의 난소 성숙을 유도하기에 충분하며11 새우와 게의 절제된 안자즙은 여러 번의 탈피 후에 재생될 수 있음이 밝혀졌다28. Scylla spp.에는 4개의 난소 발달 단계가 기록되어 있습니다: i) 미성숙(1기), ii) 조기 성숙(2기), iii) 미성숙(3단계), iv) 완전 성숙(4단계)29,30. 미성숙 난소 단계는 미성숙 여성에서 발견됩니다. 사춘기 털갈이와 짝짓기 후, 미성숙 난소는 발달을 시작하고 산란하기 전에 마침내 성숙 (4 단계)31.
눈썹 절제 프로토콜은 머드 크랩 어미의 발달과 종자 생산에 필수적입니다. 세계 식품 시장에서는 근육 함량이 높은 게보다는 완전히 성숙한 난소 (4 단계)를 가진 성숙한 머드 크랩이 소비자에 의해 선호되므로 큰 수컷보다 상업적 가치가 높습니다. 진흙 게의 눈썹 절제에 대한 완전한 프로토콜은 없습니다. 이 작업의 안자 절제 프로토콜은 완전히 마취된 게를 사용하여 스트레스를 최소화하고 게에 물린 사람의 신체적 부상을 최소화합니다. 이 프로토콜은 쉽고 비용 효율적입니다. 여기에서 우리는 생식선의 성숙을 유도할 수 있는 Scylla spp.의 안자 절제에 대한 프로토콜을 제시합니다. 두 가지 안자 절제 기술(소작 및 수술)을 테스트하고 암컷 진흙 게의 생식선 발달 속도를 기반으로 효율성을 비교했습니다.
프로토콜
이 프로토콜은 말레이시아 실험동물과학협회(Laboratory Animal Science Association of Malaysia)에서 규정한 과학적 목적을 위한 동물 관리 및 사용에 대한 말레이시아 실행 강령을 따릅니다. 실험 샘플의 희생은 National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978)에 따라 수행되었다. 성적으로 미성숙한 암컷 머드 크랩(오렌지 머드 크랩 S. olivacea)은 말레이시아 세티우 습지의 현지 시장(5°66′62′′N, 102°72′33′′E)에서 수집되었습니다. 머드 크랩 종은 형태학적 특성에 기초하여 확인되었다1.
1. 시료 채취 및 소독
- 건강하고 활동적이며 미숙한 암컷 머드 크랩을 수집합니다(그림 1).
참고: 미숙아 암컷 게는 80-85mm의 CW 범위와 함께 삼각형 및 밝은 색상의 복부 플랩을 가지고 있습니다. - 게를 염소 처리 된 수돗물 (담수)로 씻어 파편과 삼투 성 기생충을 제거하십시오.
- 게를 150ppm 포름알데히드에 20ppt 염분과 함께 30분 동안 담급니다.
- 포름알데히드 처리 동안 에어스톤으로 지속적이고 부드러운 통기를 유지하십시오. 폭기 소스는 중앙 폭기 라인 또는 수족관 폭기 펌프에서 나올 수 있습니다.
- 흐르는 바닷물로 게를 씻어 잔류 포름알데히드를 제거합니다.
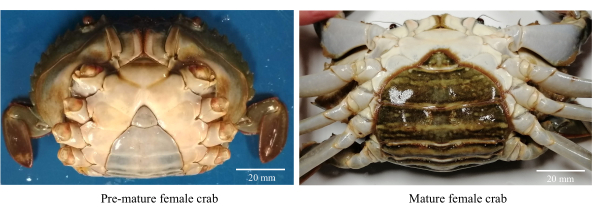
그림 1: 성적 성숙 단계를 식별하는 데 사용되는 암컷 머드 크랩의 복부 형태. 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
2. 순응
- 소독 된 각 암을 별도의 32L 원형 탱크에 옮깁니다.
- 20 ppt 염분에서 3 일 동안 암컷을 키우고 게 체중의 약 4 % -5 %에서 잘게 잘린 해양 물고기로 하루에 두 번 (아침 09:00 오전 및 저녁 20:00 오후) 계속 먹이십시오.
- 아침 먹이기 전에 사이펀으로 과잉 사료와 먹지 않은 사료를 제거하십시오.
- 게 사육 바닷물 (20 ppt)의 10 %를 매일 교환하십시오.
3. 성적 성숙을 위한 유도 탈피
- 멸균 된 가위를 사용하여 수영 다리를 제외한 모든 다리를 자릅니다.
- 스쿱 그물로 게를 잡고 게를 조심스럽게 잡습니다. 먼저 두 cheliped를 자른 다음 가위를 사용하여 두 번째 관절에서 걷는 다리를 자릅니다. 게는 손상된 부속물을 자동으로 자동 조정합니다. 사지 자율절제술에는 마취가 필요하지 않습니다.
- 사지 자율절제술 직후 민물에서 게를 씻으십시오.
- 사지가 자율적으로 절단된 게를 구멍이 뚫린 플라스틱 바구니(28cm L x 22cm W x 7cm H)에 개별적으로 옮기고 유리 섬유 탱크(305cm L x 120cm W x 60cm H)에 넣습니다.
알림: 두 개의 바구니를 묶고 함께 고정할 수 있습니다. 상단 바구니는 게가 바구니에서 빠져 나올 수 없도록 덮개로 사용됩니다. - 염도가 20ppt이고 수심이 10cm 이상인 재순환 양식 시스템(RAS)을 사용하여 전체 플라스틱 바구니가 잠기도록 합니다.
- 사지가 자율적으로 조절된 암컷 게에게 게 체중의 5%-7%로 하루에 두 번 잘게 잘린 해양 물고기를 계속 먹이십시오.
- 털갈이를 통해 성적으로 성숙할 때까지 게를 키웁니다(35일).
참고: 유도 탈피는 상업적 난소 성숙 및 야생 성숙 암컷 진흙 게로 종자 생산을 위해 건너뛸 수 있습니다. 야생에서 수확 된 성숙한 암컷은 순응해야하며 냉담한 마취와 후속 안구 절제를 직접 받아야합니다.
4. 마취
- CW >86 mm의 짙은 색의 타원형 복부 플랩이있는 성적으로 성숙한 여성을 선택하십시오 (그림 1).
- 국자 그물로 게를 잡아 마취를 위해 작은 수족관에 개별적으로 보관하십시오.
- 순응 기간 5분 후 각 수족관에 2mL/L의 2-페녹시에탄올(2-PE)을 넣고 15분간 마취 치료를 합니다.
- 게가 자발적인 움직임의 부족으로 완전히 마취되었는지 확인하십시오.
5. 눈썹 절제
- 소작 기술
- 테이블 위와 열린 공간에서 모든 절차를 수행하십시오.
- 나무 또는 플라스틱 손잡이가 있는 납작한 머리 니켈-강철 금속 막대(예: 드라이버)를 잡고 젖은 면 수건으로 손잡이를 덮습니다.
- 오토클레이브에서 두 개의 스테인리스 수술용 집게를 소독합니다.
- 스프레이 병에 70% 에탄올을 준비하고 토치 및 빨간색 뜨거운 드라이버와 같은 화재 관련 소스에서 멀리 두십시오. 티슈 페이퍼를 사용할 수 있도록 준비하십시오.
알림: 에탄올은 가연성이 높습니다. 화기로부터 안전 거리를 유지하십시오. - 토치를 가스 실린더(부탄)에 단단히 연결합니다.
주의 : 토치와 가스 실린더의 지침을 따르십시오. 가스 실린더와 연결할 때 토치가 꺼져 있는지 확인하십시오. 가스 실린더에 언급된 모든 화재 안전 예방 조치를 읽고 따르십시오. - 뜨거운 물체로 인한 부상을 방지하기 위해 두꺼운 면장갑을 착용하십시오.
- 금속 막대가 밝은 빨간색이 될 때까지 금속 막대의 끝을 토치의 불에 가두십시오.
- 마취 된 게를 젖은 면수건으로 덮으십시오.
알림: 불필요한 손상을 방지하기 위해 게의 안테나를 덮으십시오. - 멸균 된 집게로 게의 한쪽 눈을 잡으십시오.
알림: 처음 사용할 때는 오토클레이브에서 집게를 소독하고 다른 게에 사용할 때는 70% 에탄올을 사용하여 소독합니다. - 빨갛게 달궈진 금속 납작한 끝을 게의 눈에 대고 눈줄기가 주황색 또는 붉은 주황색으로 변할 때까지 약 10-15초 동안 약간 누릅니다. 이 단계를 수행할 때 인접 구조물의 손상을 방지하기 위해 주의하십시오.
참고: 소작 방법에 따라 안자 절제술을 시행하려면 두 사람이 필요합니다: 한 사람은 게를 잡고 다른 한 사람은 절제 절차를 수행합니다. - 게 사이에 교차 오염이 없도록 70% 에탄올 스프레이로 집게를 소독하십시오.
알림: 잠재적인 화재 위험을 방지하기 위해 5% 에탄올을 사용하여 소독하기 전에 겸자가 냉각되었는지 확인하기 위해 안자 절제 절차 후 최소 70분 동안만 이 단계를 수행하십시오. - 모든 게에 대해 눈썹 절제를 수행한 후 뜨거운 니켈강 금속 막대(드라이버)를 수돗물에 담그십시오.
- 재사용하기 전에 수건을 소독하십시오. 시간을 절약하기 위해 여러 수건을 사용할 수 있습니다.
알림: 수건을 수돗물로 씻고 30ppm의 염소 처리된 물에 5분 동안 담그십시오. 그런 다음 수건을 다시 수돗물로 씻고 1g/L 티오황산나트륨 용액에 담근다. - 토치를 끈 후 안전한 장소에 보관하고 환경 온도(약 30분)로 돌아올 때까지 기다렸다가 분리하십시오.
- 수술 기법
- 환기가 잘되는 곳에서 절차를 수행하십시오.
- 오토클레이브에서 두 개의 수술용 가위와 집게를 소독합니다.
- 70% 에탄올 50mL를 100mL 유리 비커에 붓습니다.
- 두꺼운 면장갑을 착용하십시오.
- 마취 된 게를 잡고 젖은 면수건으로 덮으십시오.
- 멸균 된 집게로 게의 한쪽 눈을 잡으십시오.
- 멸균 된 수술 용 가위를 사용하여 눈썹을 신속하게 잘라냅니다.
참고: 게의 상처 부위에서 체림프가 손실될 수 있습니다. - 사용 후 가위와 집게를 70% 에탄올에 담그고 티슈 페이퍼로 건조시킨 후 재사용하십시오.
6. 마취 후 관리
- 20 ppt 여과 된 바닷물을 준비하고 연속 통기로 오버 헤드 탱크에 보관하십시오.
- 중력 물 흐름을 위해 오버 헤드 탱크와 유연한 파이프를 연결하십시오.
- 눈썹 절제 직후 게를 바구니에 넣고 게를 오버헤드 탱크에서 흐르는 바닷물(주변 수온: 28°C)에 노출시킵니다.
- 바닷물이 계속 흐르도록 하고 게가 자발적으로 움직일 수 있을 때까지 모니터링하여 마취에서 회복되었음을 나타냅니다.
알림: 해수는 지하 탱크에서 준비할 수 있으며 물의 흐름을 위해 수중 워터 펌프를 사용할 수 있습니다. - 추가 관찰을 위해 30 분 동안 수족관에 통기가있는 20 ppt 바닷물에 게를 개별적으로 보관하십시오.
참고: 회수된 게는 후속 어미 배양 과정에서 개별적으로 배양됩니다.
7. 난소 성숙 관찰
- 어미의 가축 사육
- 다 자란 게를 개별 32L 원형 탱크로 옮깁니다.
- 잘게 잘린 해양 물고기 (-20 ° C에서 냉동)를 하루에 두 번 (아침 09:00 오전 및 저녁 20:00 pm) 계속 먹이고 아침 먹이기 전에 먹지 않은 사료를 제거하십시오.
- 20 ppt 염분으로 30 일 동안 개별적으로 새끼를 낳습니다.
- 대변을 제거하고 매일 바닷물 (20 ppt)의 10 %를 교환하십시오.
- 해부
- 해부 트레이, 가위, 집게를 70% 에탄올로 청소합니다.
- 2-PE 침지 마취 방법으로 여성을 개별적으로 마취합니다.
- 생식선 단계를 확인하기 위해 안자 절제술을 거치지 않은 새로 성숙한 암컷(미숙아 암컷을 털갈이 후)을 무작위로 선택합니다.
- 눈줄기가 절제된 모든 실험 암컷을 개별적으로 희생하고 생식선 성숙 단계를 식별합니다. 날카로운 멸균 송곳을 사용하여 게의 흉부 신경절을 파괴하십시오. 먼저 갑각을 제거한 다음 난소가 보이도록 간췌장을 제거합니다. 난소 색을 관찰하고 난소 성숙 단계를 확인합니다(그림 2).
- 난소 성숙 단계 식별
- 육안으로 또는 실체 현미경으로 난소 색을 관찰하십시오.
- 착색에 기초한 난소 성숙 단계확인 30: 미성숙(단계-1)은 반투명 또는 크림색의 백색을 나타내고; 초기 성숙 (2 단계)은 옅은 황색을 띤다. (iii) 성숙 전 (3 단계)은 황색 내지 밝은 주황색을 나타낸다. (iv) 완전히 성숙한(4단계) 짙은 주황색 내지 붉은색을 나타낸다.
결과
생식선 성숙
크림색의 흰색 난소 조직(미성숙 난소, 1기)은 안자 절제술을 수행하기 전에 해부된 암컷(n = 6)의 100%에서 발견되었습니다(그림 2). 눈썹 절제술을 받은 암컷 게(n=63; 소작 기법을 적용한 암컷 31마리, 수술 기법을 사용한 암컷 32마리)의 생식선 성숙률은 개별 사육 30일 후 안대 절제술을 받지 않은 암컷 게(n=31)에 비해 더 높았다(그림 3<...
토론
이 프로토콜은 머드 크랩 Scylla spp.의 안구 절제를 위해 개발되었으며 생식선 성숙을 유도하는 효율적인 방법으로 적용할 수 있습니다. 이 프로토콜은 머드 크랩의 상업적 난소 성숙을 위해 쉽게 복제할 수 있으며 머드 크랩 종자 생산에서 잠복기(산란에서 다른 산란까지의 시간)를 줄이기 위해 구현할 수 있습니다.
갑각류(즉, 민물 새우, 해양 새우)의 눈썹 절제는 일반?...
공개
저자 중 누구도 이해 상충이 없습니다.
감사의 말
이 연구는 말레이시아 테렝가누 대학교(Universiti Malaysia Terengganu)의 열대 양식 및 수산 연구소(Institute of Tropical Aquaculture and Fisheries)의 인증을 받은 말레이시아 HICoE(Higher Institution Centre of Excellence) 프로그램에 따라 말레이시아 교육부의 지원을 받았습니다(투표 번호 63933 및 투표 번호 56048). 우리는 Universiti Malaysia Terengganu 및 Sayap Jaya Sdn. Bhd. 민간 파트너십 연구 보조금 (Vot. No. 55377)을 통해 . Universiti Sains Malaysia에서 Khor Waiho 및 Hanafiah Fazhan에 이르는 겸임 학술 펠로우 직책도 인정됩니다.
자료
| Name | Company | Catalog Number | Comments |
| Aeration tube | Ming Yu Three | N/A | aquarium and pet shop |
| Airstone | Ming Yu Three | N/A | aquarium and pet shop |
| Autoclave machine | HIRAYAMA MANUFACTURING CORPORATION | N/A | MADE IN JAPAN |
| Bleaching powder (Hi-Chlon 70%) | Nippon Soda Co.Ltd,Japan | N/A | N/A |
| Blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Circular tank (32L) | BEST PLASTIC INDUSTRY SDN. BHD. | N/A | N/A |
| Cotton hand gloves (thick) | MR D.I.Y. Group Berhad | N/A | N/A |
| Cotton towel | MR D.I.Y. Group Berhad | N/A | N/A |
| Digital thermometer | Hanna Instrument | HI9814 | Hanna Instruments GroLine Hydroponics Waterproof pH / EC / TDS / Temp. Portable Meter HI9814 |
| Digital Vernier Caliper | INSIZE Co., Ltd. | N/A | |
| Dissecting tray | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Dropper bottle/Plastic Pipettes Dropper | Shopee Malaysia | N/A | N/A |
| Ethanol 70% | Thermo Scientific Chemicals | 033361.M1 | Diluted to 70% using double distilled water |
| Fiberglass tank (1 ton) | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Fine sand | N/A | N/A | collected from Sea beach of Universiti Malaysia Terengganu |
| First Aid Kits | Watsons Malaysia | N/A | N/A |
| Flat head nickel steel metal rod (Screw driver) | MR D.I.Y. Group Berhad | N/A | N/A |
| Formaldehyde | Thermo Scientific Chemicals | 119690010 | |
| Gas cylinder (butane gas) for blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Gas lighter gun (long head) | MR D.I.Y. Group Berhad | N/A | N/A |
| Glass beaker (100 mL)) | Corning Life Sciences | 1000-100 | |
| Ice bag | Watsons Malaysia | N/A | N/A |
| Perforated plastic baskets | Eco-Shop Marketing Sdn. Bhd. | N/A | N/A |
| PVC pipe 15mm | Bina Plastic Industries Sdn Bhd (HQ) | N/A | N/A |
| Refractometer | ATAGO CO.,LTD. | ||
| Refrigerator | Sharp Corporation Japan | N/A | Chest Freezer SHARP 110L - SJC 118 |
| Scoop net | MR D.I.Y. Group Berhad | N/A | |
| Seawater | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Siphoning pipe | MR D.I.Y. Group Berhad | N/A | N/A |
| Spray bottle | Mr. DIY Sdn Bhd | N/A | N/A |
| Stainless surgical forceps | N/A | N/A | N/A |
| Stainless surgical scissors | N/A | N/A | N/A |
| Submersible water pump | AS | N/A | model: Astro 4000 |
| Tincture of iodine solution (Povidone Iodine) | Farmasi Fajr Sdn Bhd | N/A | N/A |
| Tissue paper | N/A | N/A | |
| Transparent plastic aquarium | Ming Yu Three | N/A | aquarium and pet shop |
| Waterproof table | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
참고문헌
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H., et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K., et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y., et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production. Aquaculture Reports. 18, 100432 (2020).
- Waiho, K., et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R., et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T., et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S., et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G., et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I., et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N., et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N., et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C., et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H. -. Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q., et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A., et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8, (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K., et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus. Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S. -. K., Lee, S. -. G., Lee, J. -. M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity. Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X., et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C., et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C., et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 130 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H., et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Erratum
Formal Correction: Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs
Posted by JoVE Editors on 5/26/2023. Citeable Link.
An erratum was issued for: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs. The Introduction, Protocol, Discussion and References were updated.
The forth sentence in the third paragraph of the Introduction has been updated from:
The eyestalk ablation protocol in this work minimizes stress by using fully sedated crabs and minimizes physical injury to personnel from crab bites.
to:
The eyestalk ablation protocol in this work minimizes stress by using fully anesthetized crabs and minimizes physical injury to personnel from crab bites.
The start of the Protocol has been updated from:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia. The sacrifice of the experimental samples was done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Sexually pre-mature female mud crabs (orange mud crab S. olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
to:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia and was approved by the Universiti Malaysia Terengganu's Research Ethics Committee (Animal ethics approval number: UMT/JKEPHMK/2023/96). The sacrifice of the experimental samples was done according to the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Sexually pre-mature female mud crabs (orange mud crab Scylla olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
Section 4 of the Protocol has been updated from:
4. Cold-shock anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for cold shock anesthesia.
- Prepare 2 L of 4 °C to 1 °C seawater (20 ppt) in a transparent plastic aquarium. Maintain the temperature using (−20 °C) ice bags for cold shock anesthesia.
NOTE: Check the temperature with a digital thermometer. - Immerse the crab in the 4 °C seawater until sedated (about 3−5 min).
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement. The legs and chelipeds joints will still show minor movements when touched with forceps.
to:
4. Anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for anesthesia.
- After 5 min of acclimatization period, add 2-phenoxyethanol (2-PE) at 2 mL/L into each aquarium and allow 15 min of anesthesia treatment.
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement.
Section 5 of the Protocol has been updated from:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized (sedated) crab with a wet cotton towel.
NOTE: Cover all the tentacles of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
- After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Prepare the tincture of iodine solution in a dropper bottle.
NOTE: Tincture of iodine (iodine tincture or weak iodine solution) is made up of 2%-7% elemental iodine and potassium iodide, or sodium iodide, dissolved in ethanol and water. - Wear thick cotton gloves.
- Hold the sedated crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
- Apply two to three drops of iodine tincture to the wounded part of the eyestalk immediately after cutting it off.
NOTE: Tincture of iodine is used for healing and to prevent infection.
to:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle and keep it away from any fire-related sources, such as blow torch and red hot screwdriver. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized crab with a wet cotton towel.
NOTE: Cover the antennae of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color. Be careful when conducting this step to avoid damage to adjacent structures.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
NOTE: Only perform this step at least waiting for 5 min after the eyestalk ablation procedure to ensure the forceps are cooled down before disinfection using 70% ethanol to prevent potential fire hazards. - After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Wear thick cotton gloves.
- Hold the anesthetized crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
Step 7.2.2 of the Protocol has been updated from:
Sedate the females individually with the cold shock anesthesia method.
to:
Anesthetize the females individually with the 2-PE immersion anesthesia method.
The Discussion has been updated from:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,40,41. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,42. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,42,43. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,44. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique requires an additional step of disinfection using iodine. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,45,46.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. The application of a tincture of iodine can prevent infection of the wounded part. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,47. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,48,49.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,50,51. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,52. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
to:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Anesthesia via immersion in 2-phenoxyethanol was used as it is comparable to the use of tricaine methanesulfonate (MS-222) in arthopods but cheaper and does not require the use of additional buffer40. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,41,42. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,43. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,43,44. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,45. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique takes time for the wound to heal and this would allow for chance of infection. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,46,47.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,48. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,49,50. In addition, future research on pain assessment following eyestalk ablation on mud crabs is recommended to highlight the change in behaviours associated with pain and stress, as evident in freshwater prawn Macrobrachium americanum51.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,52,53. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,53. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
The References have been updated from:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
to:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기더 많은 기사 탐색
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유