Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Assaying the Ability of Diffusible Signaling Molecules to Reorient Embryonic Spinal Commissural Axons
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This assay assesses the ability of a signaling molecule, here Bone Morphogenetic Protein 7 (BMP7), to reorient commissural axons. An explant of embryonic dorsal spinal cord is cultured adjacent to an aggregate of COS cells secreting the candidate growth factors. Reoriented commissural axons growing within the explant are visualized by immunohistochemistry.
Streszczenie
Dorsal commissural axons in the vertebrate spinal cord1 have been an invaluable model system in which to identify axon guidance signals. Here, we describe an in vitro assay, "the reorientation assay", that has been used extensively to study the effect of extrinsic and intrinsic signals on the orientation of commissural axons2. This assay was developed by numerous people in the laboratories of Jane Dodd, Thomas Jessell and Andrew Lumsden (see acknowledgements for more details) and versions of this assay were used to demonstrate the reorientation activities of key axon guidance molecules, including the BMP chemorepellent in the roof plate3,4 and the chemoattractive activities of Netrin15 and Sonic Hedgehog (Shh)6 in the floor plate in the spinal cord.
Explants comprising 2-3 segments of the dorsal two-thirds of spinal cord are dissected from embryonic day (E) 11 rats and cultured in three dimensional collagen gels7. E11 dorsal spinal explants contain newly born commissural neurons, which can be identified by their axonal expression of the glycoprotein, Tag18. Over the course of 30-40 hours in culture, the commissural axon trajectory is recapitulated in these dorsal explants with a time course similar to that seen in vivo. This axonal trajectory can be challenged by placing either test tissues or a COS cell aggregate expressing a candidate signaling molecule in contact with one of the lateral edges of the dorsal explant. Commissural axons extending in the vicinity of the appended tissue will grow under the influence of both the endogenous roof plate and signals from the ectopic lateral tissue. The degree to which commissural axons are reoriented under these circumstances can be quantified. Using this assay, it is possible both to examine the sufficiency of a particular signal to reorient commissural axons3,4 as well the necessity for this signal to direct the commissural trajectory9.
Protokół
Part 1: Preparation of Aggregates of Transfected COS Cells using Hanging Drops
- Seed COS-7 (COS) cells in 35mm culture dishes. When they reach 80% confluency, transfect 1μg of the expression plasmid into the cells using Lipofectamine2000 according to the manufacturer s protocol.
- To prepare hanging drops, aspirate the transfection medium and rinse the transfected COS cells with 1ml of 1x Phosphate Buffered Solution (PBS). Treat cells with 0.5ml of enzyme-free cell dissociation medium for 15 minutes. Add 1ml of a solution of Opti-MEM + 1x Penicillin/Streptomycin/Glutamine (P/S/G) + 10% Fetal Bovine Serum (FBS) to stop the reaction.
- Triturate the cells to remove them from the surface of the culture dish and transfer into a 15ml conical tube. Spin for 2 minutes at 2000K to pellet the cells. Remove supernatant and resuspend pellet in 100μl of Opti-MEM + 1x P/S/G + 10% FBS.
- Spot multiple 20μl drops on the inside of a 35mm culture dish lid, invert lid and place on top of the bottom of the dish and leave in a 37°C incubator for several hours until the cells aggregate.
Part 2: Preparation of Dorsal Spinal Cord Explants
- Dissect E11 rat embryos from the uterus of the mother and keep on ice in L15 medium until needed.
- With a sharpened tungsten needle, remove a 4-6 segment section from the trunk of each embryo, from the region immediately below forelimb bud. Using a plastic pipette, collect the tissue pieces in one well of a 4-well Nunc dish and keep on ice.
- When all the embryo segments have been dissected, use the plastic pipette to transfer the pieces into a solution of 1ml L15 + 1mg dispase in a second well of the Nunc dish. Incubate at room temperature for no longer than 5 minutes. Do not over incubate the tissue pieces with dispase.
- During the incubation, add 0.5ml of heat inactivated normal goat serum (HIGS) to about 10ml of L15 into a Petri dish and swirl to mix. Transfer 1ml of this solution into a third well of the Nunc dish and transfer the tissue pieces into this solution when the incubation is over. Keep on ice. Note: the subsequent dissection will be easier if the tissue pieces are permitted to "rest" on ice for an hour.
Part 3: Priming Collagen
- Add 40μl 10x Minimal Essential Medium to 360μl collagen, mix rapidly and thoroughly by flicking or briefly vortexing the tube. Spin down rapidly in a picofuge. The solution will be yellow. Keep on ice as much as possible.
- Add enough 0.8M sodium bicarbonate (NaHCO3) to turn the collagen solution slightly orange (see note below). Again, mix rapidly and thoroughly by flicking, and spin down briefly in a picofuge. If the solution remains pink after mixing, you have added too much NaHCO3 and will need to start again with a fresh tube of collagen.
- At this point the collagen solution is primed. It will remain liquid on ice, but will solidify within about 5 minutes (turning pink) when brought to room temperature.
- Spot 20μl of collagen in each well of a 4-well Nunc dish. Using the tip of your pipette, spread out the collagen to form a small "pad". Let the collagen set at room temperature.
Note: The exact amount of NaHCO3 will need to be titrated for each batch of collagen. Start low (11μl) and then add 0.5-1μl stepwise until the solution is a slight shade of orange. The "right" amount is usually 1ul less than the smallest amount of NaHCO3 that would turn the collagen pink. The amount of NaHCO3 does not scale linearly up or down.
Part 4: Dissection of Dorsal Spinal Cord Explants and Positioning of Them with COS Cell Aggregates in Collagen Matrix
- Using a freshly sharpened tungsten needle and a pair of fine forceps (#5 or #55), remove the mesoderm surrounding the spinal cord.
- Cutting parallel to the floor plate with the tungsten needle, carefully remove the ventral fifth of the spinal cord.
- Bisect the dorsal spinal explant, making sure that edges are as cleanly cut as possible. Re-trim the edges, if necessary. Transfer each dorsal spinal explant onto separate collagen pads using a mouth pipette fitted with a glass capillary tube pulled to approx. 0.5mm in diameter.
- Cut the aggregate of transfected COS cells into squares of approximately the same width as the lateral edge of the dorsal spinal explant.
- Transfer one of these squares onto the collagen pad with the dorsal spinal explant, using a mouth pipette.
- With the mouth pipette, aspirate off any excess liquid using the mouth pipette. Do not over aspirate.
- Apply 4μl of primed collagen onto the pad, and, using the tungsten needle, move the collagen over the explants without directly touching the tissue. By moving the tungsten needle in the collagen surrounding the explants, orient the explants so that the COS cells aggregate is adjacent to the lateral edge of the dorsal spinal explant.
- After the collagen has set, repeat the application of 4μl of collagen to ensure that the explants are completely encased in collagen.
- In a tissue culture hood, add 0.5ml of Opti-MEM + 1xP/S/G and culture for 30-40 hours.
- Fix explants for 45 minutes with 0.5ml 4% paraformaldehyde in 1xPBS, keeping the plate on ice.
- Wash twice with 0.5ml of a blocking solution of 1xPBS + 0.1% Triton-X100 +1% HIGS (PBTN). Block at 4°C for at least a couple of hours, an overnight incubation is preferable and then process by immunohistochemistry.
Part 5: Visualization of Commissural Axons by Immunohistochemistry
- After blocking in PBTN, cut the explants out of the 4 well plate with forceps and place in a 48-well dish.
- Add 200μl of primary antibodies to each well and leave overnight at 4°C. To visualize commissural axons, use a 1:6 solution of mouse anti-Tag1 antibody. To determine whether the transfection was successful, also include a primary antibody against either the signaling molecule being tested, or the relevant epitope if the signaling molecule has been epitope tagged.
- Wash each sample for 5 x 1 hour in PBTN at 4°C.
- Add 200μl of secondary antibody to each well and leave overnight at 4°C. If anti-Tag1 antibodies were used as the primary antibody, use a goat anti mouse IgM secondary antibody coupled to either FITC or Cy3. If using light sensitive secondary antibodies coupled to FITC or Cy3, keep the 48-well dish covered in foil from this point on.
- Wash each sample 5 x 1 hour each in PBTN at 4°C.
- Transfer explant to a 3-well depression slide, remove excess liquid, mount in Vectashield medium and coverslip. Store in a light safe box at either 4°C or -20°C.
Part 6: Representative Images of Explants
In a successful experiment, the explant and COS aggregate will remain adjacent to one another and not drift apart as the collagen sets. There will be minimal overgrowth of axons; significant axonal overgrowth indicates that the dorsal spinal explant was cultured for too long. There will be no blebs growing from the explant; blebbing occurs if the tissue comes into direct contact with the culture medium.
The ability of the signaling molecule to reorient axons is assessed using confocal microscopy. The extent of axon reorientation can be quantified by measuring the average angle of reorientation of the axons closest to the COS cell aggregate3. As shown in Figure 1, COS cells expressing myc-tagged BMP7 (blue) reorient Tag1+ commissural axons (green) away from the source of BMP7 (Figure 1B) similar to the manner to which an explant of roof plate repels commissural axons (Figure 1A). COS cells expressing a control vector have no effect on the orientation of commissural axons3,4. These explants were also labeled with antibodies against the transcription factor Isl1/2 (red), which decorates motor neurons and dorsal interneurons. This result was the first indication that a member of the BMP family mediates the activity of the roof plate chemorepellent3.
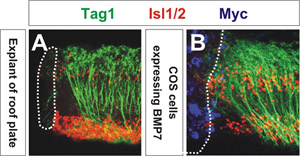
Figure 1: Please click here to see a larger version of figure 1.
Dyskusje
The critical factors that determine success in performing this assay are first, the tissue should not be treated with dispase for too prolonged a period, such treatment will result in the tissue becoming very sticky and having decreased viability. Two, the collagen must be perfectly primed and kept on ice as much as possible. It will become increasingly difficult to handle if it starts to set, i.e. turn pink. Three, the tungsten needle must always be kept very sharp.
Podziękowania
This protocol was developed in the laboratories of Jane Dodd, Thomas Jessell and Andrew Lumsden. Many people, including Konrad Basler, Ann Calof, Thomas Edlund, Phil Hamilton, Domna Karagogeos, Ariel Ruiz i Altaba and Toshiya Yamada determined how to culture explants of spinal cord in collagen. Marysia Placzek and Marc Tessier-Lavigne pioneered the technique of using axon growth from in vitro explants as a means of identifying axon guidance molecules. Work in the Butler laboratory is supported by grants from the March of Dimes and R01 NS063999 from NIH/NINDS.
Materiały
| Name | Company | Catalog Number | Comments |
| Type 1 rat tail collagen | BD Biosciences | 354236 | |
| COS-7 cells | ATCC | CRL-1651 | |
| 10x Minimal Essential Medium | Invitrogen | 11430-030 | |
| Opti-MEM | Invitrogen | 51985-034 | |
| L15 | Invitrogen | 11415-064 | |
| Lipofectamine 2000 | Invitrogen | 1166-8019 | |
| Fetal bovine serum | Mediatech, Inc. | 35-016-CV | |
| Cell dissociation solution | EMD Millipore | S-004-C | |
| Trypsin 0.25% EDTA | Invitrogen | 2520-0056 | |
| Sodium Bicarbonate | Sigma-Aldrich | S6297 | |
| Triton X-100 | Sigma-Aldrich | X100 | |
| Pen/Strep/Glutamate | Invitrogen | 10378-016 | |
| Paraformaldhyde | Baker/VWR | JTS898-7 | |
| Dispase | Roche Group | 10241750001 | |
| mouse anti Tag1 IgM (4D7) | Developmental Studies Hybridoma Bank | ||
| mouse anti Myc IgG (9E10) | Santa Cruz Biotechnology, Inc. | sc-40 | |
| goat anti mouse IgM FITC | Jackson | 115-095-075 | |
| goat anti mouse Fcy Cy3 | Jackson | 115-165-071 | |
| Goat serum | Invitrogen | 16210064 | |
| Vectashield | Vector Laboratories | H-1000 | |
| 4 well Nunclon dish | Nalge Nunc international | 62407-068 | |
| 3 well depression slide | VWR international | 48339-009 | |
| 48 well dish | Falcon BD | 62406-195 | |
| Aspirator tuve assembly | FHC, Inc. | 30-32-0 | |
| #55 Dumont forceps | Fine Science Tools | 11252-20 | |
| #5 Dumont forcept | Fine Science Tools | 11255-20 | |
| Needle Holder | Fine Science Tools | 26016-12 |
Odniesienia
- Altman, J., Bayer, S. A. . The development of the rat spinal cord. , (1984).
- Placzek, M. Tissue recombinations in collagen gels. Methods in molecular biology. , 325-335 (2008).
- Augsburger, A., Schuchardt, A. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 24, 127-141 (1999).
- Butler, S. J., Dodd, J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 38, 389-401 (2003).
- Kennedy, T. E., Serafini, T. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 78, 425-435 (1994).
- Charron, F., Stein, E. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 113, 11-23 (2003).
- Lumsden, A. G., Davies, A. M. Chemotropic effect of specific target epithelium in the developing mammalian nervous system. Nature. 323, 538-539 (1986).
- Dodd, J., Morton, S. B. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1, 105-116 (1988).
- Yamauchi, K., Phan, K. D., Butler, S. J. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 135, 1119-1128 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone