Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
CRC Organoid Culture: A Method to Obtain 3D Organoids from Colorectal Cancer Cells
W tym Artykule
Overview
This video describes the detailed protocol for generating 3D organoids from murine-model derived colorectal cancer cells by propagating them on artificial extracellular matrix coated plates. These CRC organoids can be used as a model to study the primary growth of human tumor cells and metastasis.
Protokół
All procedures involving animals have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Extraction of PDXs into the Cell Suspension for the CRC Organoid Culture
Experimental procedures for dissociation of CRC PDXs are outlined in Figure 1B (step 3).
- Place the tumor fragments derived from NOD/Shi-scid IL2Rγ null (NOG) mouse on a 10 cm Petri dish.
- Mince the fragments using sterile razor blades and transfer them into a 15 mL conical tube containing 8 mL of 10% fetal calf serum (FCS)-Dulbecco's Modified Eagle's Medium (DMEM) with 80 µL of collagenase enzyme stock (see Table of Materials).
- Close the tube tightly and wrap the cap with parafilm to prevent leakage. To dissociate the minced tumor fragments into the cell suspension, agitate it gently for 2 h at 37 °C. Note that there will still be many fragments of undigested tissue remaining in the tube.
- Centrifuge the tube at 300 x g for 5 min at 4 °C and remove the supernatant. Resolve the cell pellet using 10 mL of 10% FCS-DMEM to make the cell suspension.
- To eliminate undigested tissue debris, filter the cell suspension twice using the 40 µm cell strainer set on a 50 mL conical tube. Then, centrifuge the flow-through at 300 x g for 5 min at 4 °C. Remove the supernatant and keep the pellet on ice.
2. Generation of the CRC Organoids Cultured on Artificial Extracellular Matrix
Experimental procedures for the CRC organoid culture of the colon PDXs are outlined in Figure 1B (step 4).
- Establish a culture medium suitable for the PDX-derived CRC organoids (see Table of Materials, "the CRC organoid culture medium").
- Apply 150 µL of artificial extracellular matrix (see Table of Materials) per well on a 12-well plate on ice and then incubate it for 30-60 min in a 37 °C, 5% CO2 incubator to solidify the gels.
- Suspend the cell pellet (as described in 1.5). Remove the supernatant and keep the pellet on ice) in the CRC organoid culture medium (from step 2.1) with 5% FCS to achieve adjustment to 3 x 105 cells/mL. Then, seed 1 mL of the medium onto the artificial extracellular matrix-coated plate prepared in step 1.2 and incubate overnight at 37 °C under 5% CO2. Use a hemocytometer for counting the number of tumor cells including single cells and multicellular clusters (a group of adherent cells) in the cell suspension.
NOTE: 5% FCS is used to quench the residual enzymatic activity of collagenase in this study. - Carefully collect the culture medium, including floating cells that have detached from the artificial extracellular matrix-coated plate, into a 1.5 mL centrifuge tube on the following day and centrifuge it at 1,400 x g for 5 min. Then, remove the supernatant and resuspend the pellet in 70 µL of artificial extracellular matrix on ice.
- To increase CRC cell viability, overlay the tumor cell-containing artificial extracellular matrix onto the tumor organoid cells attached to the artificial extracellular matrix-coated plate and incubate for 30 min in a 37 °C, 5% CO2 incubator to solidify the artificial extracellular matrix coating. Then, incubate it with 1 mL of the CRC organoid cell culture medium with 1% FCS at 37 °C under 5% CO2.
- Change the culture medium every second day. As the CRC organoids fill the medium, it may become necessary to change the medium daily.
Wyniki
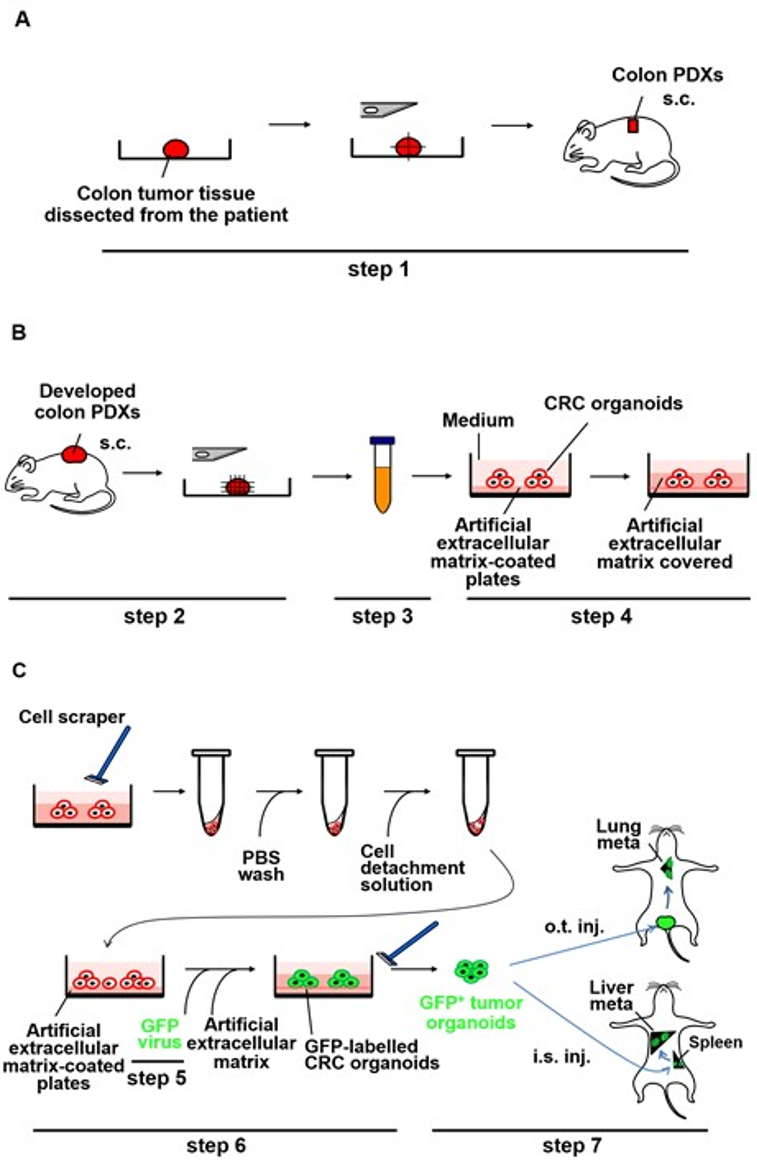
Figure 1: Schematic representation of generation of metastases by the PDX-derived CRC organoids labeled with GFP lentivirus in NOG mice. (A) Implantation of small pieces of the CRC tissue subcutaneously into NOG mice (step 1). The CRC tissue surgically dissected from the patient was cut into pieces and implanted subcutaneously into NOG mice. s.c.: subcutaneous implant...
Ujawnienia
Materiały
| Name | Company | Catalog Number | Comments |
| NOD/Shi-scid IL2Rγ null (NOG) mice | The Central Institute for Experimental Animals,Kanagawa, Japan | Breed 6-week-old male mice under germ-free and specific pathogen free conditions | |
| CRC organoid culture medium with 1% or 5% FCS | DMEM/F-12 with GlutaMAXTM supplement (Gibco #10565018) supplemented with 1% or 5% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 ng/ml hEGF and 10 µM Y27632, a ROCK inhibitor. Store at 4°C. Use within 1 month. | ||
| Matrigel basement membrane matrix | Corning | #354234 | Store aliquots at -20°C. Place on ice until use |
| Collagenase type 1 | Sigma | #C1030 | 150 mg/ml collagenase type1 in 1×PBS. Store aliquots at -20°C for up to 1 year |
| Hemocytometer | Erma | #03-202-1 | |
| DMEM/F-12 with GlutaMAXTM | Gibco | #10565018 | Store at 4°C. Warm at 37°C before use |
| 50ml conical tube | Sumitomo Bakelite | MS-57500 | |
| Microtube | Eppendorf | #0030120086 | Autoclave before use |
| 40μm cell strainer | Corning | #352340 |
This article has been published
Video Coming Soon
Source: Okazawa, Y. et al. High-sensitivity Detection of Micrometastases Generated by GFP Lentivirus-transduced Organoids Cultured from a Patient-derived Colon Tumor. J. Vis. Exp., (2018).
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone