Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Mouse Model of Surgically-induced Endometriosis by Auto-transplantation of Uterine Tissue
W tym Artykule
Podsumowanie
A description of the surgical induction of endometriosis in mice and rats by auto-transplantation of uterine tissue to the arterial cascade of the intestinal mesentery.
Streszczenie
Endometriosis is a chronic, painful disease whose etiology remains unknown. Furthermore, treatment of endometriosis can require laparoscopic removal of lesions, and/or chronic pharmaceutical management of pain and infertility symptoms. The cost associated with endometriosis has been estimated at 22 billion dollars per year in the United States1. To further our understanding of mechanisms underlying this enigmatic disease, animal models have been employed. Primates spontaneously develop endometriosis and therefore primate models most closely resemble the disease in women. Rodent models, however, are more cost effective and readily available2. The model that we describe here involves an autologous transfer of uterine tissue to the intestinal mesentery (Figure 1) and was first developed in the rat3 and later transferred to the mouse4. The goal of the autologous rodent model of surgically-induced endometriosis is to mimic the disease in women. We and others have previously shown that the altered gene expression pattern observed in endometriotic lesions from mice or rats mirrors that observed in women with the disease5,6. One advantage of performing the surgery in the mouse is that the abundance of transgenic mouse strains available can aid researchers in determining the role of specific components important in the establishment and growth of endometriosis. An alternative model in which excised human endometrial fragments are introduced to the peritoneum of immunocompromised mice is also widely used but is limited by the lack of a normal immune system which is thought to be important in endometriosis2,7. Importantly, the mouse model of surgically induced endometriosis is a versatile model that has been used to study how the immune system8, hormones9,10 and environmental factors11,12 affect endometriosis as well as the effects of endometriosis on fertility13 and pain14.
Protokół
1. Planning for live-animal surgery
- Ensure that appropriate approval has been received to work with laboratory animals.
- Order mice and allow at least one week of acclimation to their new environment.
- Female mice housed in the absence of exposure to male pheromones can stop cycling, a phenomenon referred to as the Whitten effect15,16. To keep mice cycling transfer urine-soaked male bedding to the female cage every five days. Alternatively, if open-top cages are used, place the female cage between two cages of males to keep the females cycling regularly.
- Ensure that mice are cycling by analyzing vaginal cytology daily for at least one week prior to surgery (Table 1)17.
- Use a wax pencil to create eight partitions on a glass slide so that vaginal smears from multiple mice can be collected.
- Flush the vagina with 0.2-0.25 mL normal saline or distilled water using an eyedropper. Be sure to place the eyedropper just at the vaginal orifice, as cervical stimulation with the eyedropper could cause pseudopregnancy. Place the vaginal lavage on the glass slide for analysis of cell types. Slides can be read fresh (wet) or alternatively fixed by a number of methods and examined using a standard light microscope17.
- Gather, clean and sterilize all necessary surgical equipment for a successful aseptic surgery (see Material Section)18.
- Prepare buprenorphine solution for analgesia in PBS using sterile technique to deliver 0.2 mg/kg final dose. The concentration of the buprenorphine solution should be 0.0333 mg/ml assuming that the average adult C57BL/6 mouse weighs approximately 0.025 kg and a subcutaneous injection volume of 0.15 ml per mouse. Buprenorphine can be prepared ahead of time and stored as aliquots. Note that buprenorphine is a Schedule III controlled substance requiring a DEA license and detailed inventory log.
- Prepare sterile PBS with penicillin (100 U/ml) and streptomycin (100 μg/ml).
- Synchronize estrus cycles by transferring urine-soaked male bedding to the female cages 72 hours prior to induction15.
2. Prepare the surgical area for live animal surgery
- Prepare the surgical area as previously described18.
- Prepare the preparation area by setting out electric clippers, ophthalmic ointment, and surgical scrubs.
- Prepare the surgical area by placing a recirculating hot water heating pad on the surgical area to maintain body temperature throughout the surgery. Place a sterile waterproof pad over the recirculating hot water heating pad. Arrange surgical instruments, suture, sterile glass Petri dish, biopsy punch, sterile gauze, wound clips and wound clip applicator on the sterile surgical field.
- Prepare recovery area by placing recirculating hot water heating pads halfway under an empty cage to allow mice to move away from the heat if so desired.
3. Anesthetize and prepare the mouse for surgery
- Record the weight of the mouse and determine estrus stage by evaluating the vaginal cytology.
- For induction of anesthesia, place the mouse in an empty anesthesia chamber (empty cage with solid lid containing portal for isoflurane). Turn on isoflurane non-rebreathing anesthetic system and set the vaporizer to 4% isoflurane (with an oxygen flow rate of 0.5 to 1 L/min).
- When the mouse is under anesthesia switch the isoflurane flow to a cone (30-60 ml syringe sheath) and place mouse's nose and mouth in the cone on preparation table. Adequate anesthesia can be maintained with a lower concentration of isoflurane throughout the remainder of the surgery (~2.5-3.5% isoflurane). Adequate depth of anesthesia should be determined by a negative response to toe pinch stimulus.
- Apply ophthalmic ointment to avoid drying of the eyes during surgery.
- Using small electric clippers, shave the surgery site.
- Disinfect and prepare the surgery site with three alternating swipes of chlorhexidine scrub and 70% ethanol.
- Drape animal with a sterile field.
4. Uterine ligation
- Make a small (~1 cm) midline incision using either small scissors or a scalpel blade ending 0.5 - 1.0 cm rostral to the vaginal opening.
- Insert closed scissors into the opening such that the blades are between the body wall and abdominal wall. Gently blunt dissect the area around the incision by slowly opening and closing the scissors such that the abdominal wall is sufficiently detached from the skin. Remaining visible adhesions between the abdominal wall and skin around the incision site can be carefully snipped. Failure to adequately blunt dissect the incision site will make closing the abdominal wall more difficult.
- Using small forceps, gently locate the left uterine horn. The uterus is dorsal to the intestine, which is what you will see upon first entering the incision site. In some instances it is easiest to first locate the ovary and the associated ovarian fat pad. Gently pull up on the uterine horn and slide an open forceps underneath it to serve as a retractor. If desired, note the appearance of the ovaries and uterus at this time for additional information regarding the estrus stage at induction (Table 1).
- Gently slide two 6-8 cm pieces of 5-0 black braided silk suture (without a needle) underneath the stretched uterine horn.
- Securely ligate the horn at the utero-tubual junction (just caudal to the fallopian tube) and at the utero-cervical junction (just rostral to the cervix) using a square knot at each location. Leave the ends of the suture for the moment.
- Cut out the section of uterine horn between the two ligations and place the tissue in a sterile glass Petri dish containing ~100 μL of PBS containing penicillin (100 U/ml) and streptomycin (100 μg/ml). Cut the ends of the silk suture last. If suture comes loose or there is bleeding, find the stump and tie another knot.
5. Prepare endometriotic implants from excised uterus
- While the excised uterus is being manipulated, cover the abdomen with sterile gauze and maintain hydration with sterile PBS containing penicillin and streptomycin as needed.
- Strip the excised uterine horn of fat.
- If desired, weigh the excised uterine horn.
- Open the uterine horn by inserting one blade of small scissors (14 mm blade length) into the lumen and gently sliding the scissors down the uterine horn while holding the horn with forceps.
- In the glass Petri dish, use a 2 mm biopsy punch to cut out three equal sized implants.
6. Suturing endometriotic implants in peritoneal cavity
- Place sterile gauze immediately above the incision site and thoroughly wet with sterile PBS containing penicillin and streptomycin.
- With small, smooth forceps gently find the cecum and move rostrally along the small intestine. Pull out a small (4-5 cm) section of intestine that is at least two arteries away from the cecum and arrange it like a fan on the pre-wetted gauze so that the arterial cascade of the intestinal mesentery is clearly visible. Be sure to keep the bowel moist at all times with sterile saline. Note: do not use rat-toothed forceps while handling the bowel.
- Use 6-0 black ethilon suture with a P-1, 11 mm, 3/8 circle, reverse cutting needle to gently suture one implant to an artery approximately 0.5 cm from the bowel.
- Note: The intestinal mesentery is covered by a thin layer of peritoneum. Be careful to make a clean pass through this layer while suturing around the artery. Pull suture through slowly and carefully as not to tear the peritoneum or rupture the artery.
- Complete two knots of one throw each, being careful not to tighten the suture very hard, as doing so may result in loss of blood flow and subsequent necrosis of the intestine and death. Trim the suture within 2 mm of the implant. Wet bowel again to continue to maintain hydration before moving on to next implant.
- Moving in a rostral direction, pull out the next 3-4 cm of intestine and gently replace the section that already contains an implant. Skip one or two arteries from the previous implant site and suture the next implant. Repeat for the third implant.
- Replace all of the bowel in the abdominal cavity.
7. Sham surgeries
- Sham surgeries are performed using the same steps as the endometriosis surgeries except that no tissue is sutured to the intestinal mesentery.
- Excise the left uterine horn as in step 4.
- Endometriotic implants (step 5) are not prepared in the sham surgery. The excised uterine horn can be discarded or used for other purposes if desired.
- Sutures, but no tissues, are placed around three arteries in the arterial cascade of the intestinal mesentery as in step 6.
8. Closing the surgical wound
- Ensure that all of the organs are approximately back to their anatomical position.
- Use 5-0 coated vicryl suture in a non-interlocking continuous stitch to close the abdominal wall.
- Use 9 mm wound clips to close the skin.
9. Recover animal
- Administer 0.33 mg/ml buprenorphine at 0.15 ml/25 g mouse via subcutaneous injection for a dose of 0.2 mg/kg. Buprenorphine is administered post-operatively to prevent further cardiovascular/respiratory depression that can lengthen the recovery process.
- Gently dry the mouse with kimwipes or paper towels if it has gotten wet during the surgery.
- Place animal ventral side down in cage partially atop a recirculating heated water pad until the animal is recovered and has regained sternal recumbency (within five minutes as the inhalant anesthetic quickly wears off).
10. Post-operative care
- Mice should be observed every 15 minutes until they are able to maintain sternal recumbency, then hourly until they regain their normal behavior following surgery.
- Mice should appear normal within 24 hours of surgery. Mice should be monitored daily for seven to ten days for signs of recovery and good health.
- Indications that an animal is in poor health, pain, or distress include decreased activity, self-mutilation, ungroomed appearance, or hunched posture.
- If an animal does not appear to be in good health within 24 hours of surgery, either administer buprenorphine (0.2 mg/kg) or euthanize the animal. If the animal does not improve within 8 hours of supplemental buprenorphine administration the animal should be euthanized as bowel necrosis is likely.
- Remove wound clips 7-10 days post-induction.
- Continue to monitor estrus cyclicity by examination of vaginal cytology for the duration of the experiment. Synchronize estrus cycles 72 hours prior to collection by transferring urine-soaked male bedding to the female cages as described in step 1.3.
11. Necropsy and tissue excision
- The timing of necropsy is dependent on the particular research question and is further discussed in the representative results and discussion.
- Euthanize the mouse by carbon dioxide asphyxiation.
- Collect blood by cardiac puncture using a 23 gauge needle on a 1cc syringe (if desired).
- Collect a vaginal cytology smear as described above to determine estrus stage at the time of collection17.
- Cut remaining uterine horn at utero-tubal junction and at the cervix, remove fat, weigh, and process as desired (see 11.14 and 11.15).
- Locate the black sutures around the endometriotic lesions. Photograph intact endometriotic lesions if desired.
- Carefully dissect the adhesions surrounding the endometriotic lesions with a small scissors and forceps, being careful not to lance the lesions. Work quickly and carefully to prevent RNA degradation.
- Measure and record the length and width of the endometriotic lesions using calipers.
- Excise the endometriotic lesions and place on a paper towel moistened with PBS. Remove any non-endometriotic tissue from the lesions. A dissecting microscope or magnifying glass stand can be used to aid in the dissection.
- Weigh the three fluid filled endometriotic lesions prior to removing the suture.
- Gently remove the suture from the endometriotic lesions.
- For histology, formalin fix one fluid filled endometriotic lesion for two hours followed by three thirty minute PBS washes and final storage in 70% ethanol. Dehydrate and paraffin embed.
- Lance two of the endometriotic lesions. Weigh these again. Since cyclic hormonal changes can alter the amount of cyst fluid, this gives a measure of the tissue wet-weight in addition to the weight of the cyst plus the fluid as measured in 11.10.
- For RNA isolation and gene expression studies, immediately homogenize one of the lanced endometriotic lesions (or ~20 μg uterine tissue) in lysis binding solution and store at -80°C for future RNA isolation with the RNAqueous kit (Ambion) or other method as desired.
- For future isolation of RNA, DNA, or protein, immediately snap-freeze the second lanced endometriotic lesion (or ~20 μg uterine tissue) in liquid nitrogen and store at -80°C.
Representative Results
Endometriotic lesions in the mouse model of surgically induced endometriosis morphologically and histologically resemble those observed in women. Histological analysis of endometriosis in both women and the mouse model indicates that endometriotic lesions contain endometrial glands and stroma (Figure 2A). Endometriotic lesions in mice also contain hemosiderin-laden macrophages, which are a common hallmark of endometriosis in women (Figure 2B)19.
Endometriotic lesions removed from mice three days post-induction appear inflamed and hemorrhagic (Figure 3A). After two to four weeks of growth endometriotic lesions in the mouse model are cyst-like, fluid filled and surrounded by peritoneal adhesions (Figures 3B and 3C). Compared to lesion weight at induction, fluid filled lesions were 306% and 862% larger at one and two months post-induction and lanced lesions were 51% and 172% larger, respectively (Figures 4A and 4B). We have obtained consistent fluid filled and lanced endometriotic lesion weights at one-month post-induction over five different experiments (Figure 5). At one month post-induction fluid filled (7.44±3.75 mg) and lanced (2.92±1.23 mg) endometriotic lesion weight were significantly correlated (Pearson's correlation coefficient = 0.669, p < 0.001).
Age of the mouse did not affect lesion size for mice between three and ten months of age. Neither the fluid filled or lanced endometriotic lesion weight at one month post-induction was significantly correlated with the age of the animal (r = -0.136, p = 0.380 and r = -0.063, p = 0.698, respectively).
The mouse uterus undergoes changes in size, fluid retention, cell proliferation and appearance due to the influence of steroid hormones during the estrus cycle. We compared the endometriotic lesion weight to the weight of the remaining intact uterine horn from animals in different estrus stages. We did not find a significant correlation between uterine weight and fluid filled or lanced endometriotic lesion weight at one-month post induction (r = -0.046, p = 0.765 and r = 0.232, p = 0.155, respectively).
The gene expression pattern observed in the endometriotic lesions of mice closely mirrors that reported in women with the disease5. By three days post-induction genes regulating extracellular matrix remodeling, cell adhesion, and angiogenesis are highly upregulated and many of these genes remain upregulated through one month of growth.
Figures and Tables

Figure 1. Surgical induction of endometriosis by autologus uterine tissue transfer in the mouse. The left uterine horn is ligated, excised, and opened longitudinally to expose the endometrium. Three 2 mm2 biopsies are prepared and each is sutured to an artery in the arterial cascade of the intestinal mesentery. By one month post-induction the endometriotic lesions are fluid filled and surrounded by adhesions.
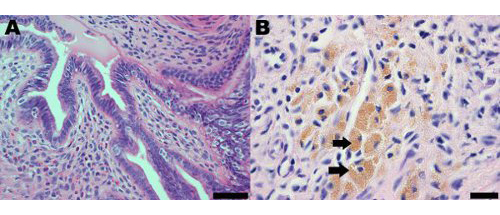
Figure 2. Hematoxylin and eosin stained section of an endometrial lesion from the mouse model of endometriosis at one month post-induction demonstrating (A) the presence of endometrial glands and stroma; scale bar = 50 μm and (B) hemosiderin-laden macrophages, some of which are indicated by arrows; scale bar = 20 μm.

Figure 3. Endometriotic lesions in the mouse model following euthanasia, either three days post-induction (A) or one month post-induction (B and C).
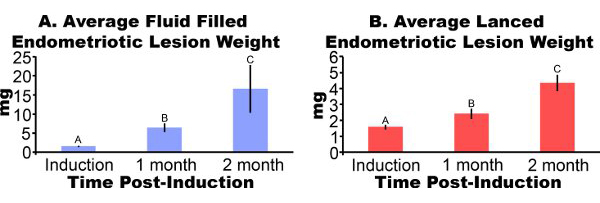
Figure 4. Endometriotic lesions from mice surgically induced to have endometriosis were excised and weighed at one or two months post-induction. Data are average±SEM. Data were log transformed and different letters indicate significance within each panel by one-way ANOVA followed by one-sided Fisher's Least Significant Difference Mulitple Comparisons. (A) Cyst like, fluid filled endometriotic lesions (N = 10, 7 or 5 for induction, one month, or two month post-induction, respectively). (B) Lanced endometriotic lesions (N = 10, 8 or 7 for induction, one month or two month post-induction respectively).
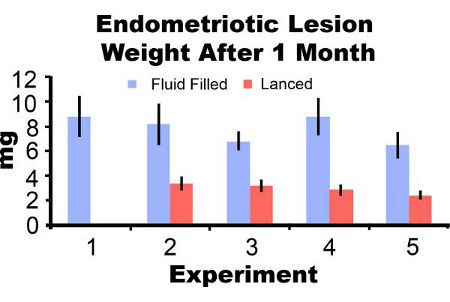
Figure 5. Endometriotic lesion wet weight with fluid and lanced at one month post-induction from five separate experiments. Data are average±SEM. Mice N=10, 6, 8, 7 and 7 for fluid filled lesions and 0, 7, 10, 8, and 8 for lanced lesions in experiment 1, 2, 3, 4, and 5, respectively.

Table 1. Observation of Estrus Stage by Vaginal Cytology and Visual Appearance of Ovaries and Uterus and Induction.
Appearance of ovary and uterus will be time dependent. The following are based on sacrifice around 8:00 am the morning of each cycle day. Further, the observations are subjective and comparing the ovary and uterine horns will be a better estimate than uterine horns only. These observations are meant to supplement the information obtained from daily vaginal cytology readings.

Table 2. Comparison of surgery in mouse and rat.
Access restricted. Please log in or start a trial to view this content.
Dyskusje
There are several critical parameters that should be noted while performing the surgical induction of endometriosis in mice. First, endometriosis is an estrogen dependent disease and as such this surgery should be performed in intact animals or alternatively in ovariectomized animals supplemented with estrogens20. Second, suturing the endometrial biopsies to the arterial cascade must be performed with extreme care. We have found that using only two relatively loose knots with one throw each keeps the biopsy in...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
No conflicts of interest declared.
Podziękowania
Special thanks to Chris Kassotis and Audrey Bailey for critical review of this manuscript and to Dr. Scott Korte, Joseph Beeman, Alison Curfman, Paul Kimball, Bridget Neibreggue, Jacob Redel, Amy Schroder, Maija Steinberg, and Stacey Winkeler for their assistance in optimization of this model in our laboratory. Funding was provided by the Clinical Biodetectives Training Grant (NIH T90) (KEP), University of Missouri Life Sciences Undergraduate Research Opportunities Program, MU Research Council, MU Research Board grants and NIH R21HD056441 (SCN).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Wax pencil | Fisher Scientific | NC9954135 | |
| Glass slide | Fisher Scientific | 12-550-433 | |
| Eyedropper | Fisher Scientific | S79383 | |
| Standard light microscope for evaluating vaginal cytology smears | |||
| Buprenorphine HCL c3 (CARJET) 10X1ml | Butler Animal Health Supply | 022891 | |
| Sterile phosphate buffered saline (PBS) | GIBCO, by Life Technologies | 14040-117 | |
| 10,000U/ml Penicillin, 10,000μg/ml Streptomycin in 0.85% NaCl | Hyclone | SV30010 | |
| Isoflurane | Abbott Laboratories | 05260-05 | |
| Isoflurane non-rebreathing anesthetic system | |||
| Recirculating hot water heating pad | |||
| 30 ml syringe sheath | Fisher Scientific | 14-823-16G | |
| Powder free sterile gloves | Fisher Scientific | 19020558 | |
| Ophthalmic ointment | Major Pharmaceuticals | 10033691 | |
| Small electrical clippers | Wahl Clipper Corp. | 9861-600 | |
| Chlorhexidine scrub | Fisher Scientific | NC9863042 | |
| 70% Ethanol | |||
| Polylined sterile field | Busse Hospital Disposables | 696 | |
| Size 3 scalpel | Fisher Scientific | 22-079-657 | |
| Number 10 scalpel blades | Fisher Scientific | 22-079-681 | |
| Small surgical scissors | Roboz Surgical Instruments Co. | RS-5850 | |
| Small serrated semi-curved forceps | Roboz Surgical Instruments Co. | RS-5135 | |
| 5-0 black braided silk suture | Ethicon Inc. | K870H | |
| Sterilized pyrex glass Petri dishes | Corning | 70160-101 | |
| 2 mm biopsy punch | Miltex Inc. | 33-31 | |
| Sterile gauze | Kendall | 1806 | |
| 6-0 black monofilament ethilon nylon suture | Ethicon Inc. | 697G | |
| Needle drivers (optional) | World Precision Instruments, Inc. | 500023 | |
| 5-0 undyed braided coated vicryl suture | Ethicon Inc. | J490G | |
| 9mm Autoclip wound clips | BD Biosciences | 427631 | |
| Autoclip applier & remover | BD Biosciences | 427630 | |
| 23G needle | BD Biosciences | 305193 | |
| 1cc syringe | BD Biosciences | 301025 | |
| 5X magnifying glass stand (optional) | Fisher Scientific | 14-648-23 | |
| 10% Buffered formalin | Fisher Scientific | SF100-4 | |
| Calipers | Roboz Surgical Instruments Co. | RS-6466 | |
| Processing/embedding cassettes | Fisher Scientific | 15-197-700A | |
| Biopsy foam pads | Fisher Scientific | 22-038-222 | |
| RNAqueous RNA isolation kit | Ambion | AM1912 | |
| Liquid nitrogen | |||
| Snap cap microcentrifuge flat top tube | Fisher Scientific | 02-681-240 | |
| Ketamine (optional) | Sigma-Aldrich | K4138 | |
| Domitor (medetomidine hydrochloride) (optional) | Tocris Bioscience | 2023 | |
| Antisedan (atipamezole) (optional) | Sigma-Aldrich | A9611 |
Odniesienia
- Simoens, S., Hummelshoj, L., D'Hooghe, T. Endometriosis: cost estimates and methodological perspective. Hum. Reprod. Update. 13, 395-404 (2007).
- Grummer, R. Animal models in endometriosis research. Hum. Reprod. Update. 12, 641-649 (2006).
- Vernon, M. W., Wilson, E. A. Studies on the surgical induction of endometriosis in the rat. Fertil. Steril. 44, 684-694 (1985).
- Cummings, A. M., Metcalf, J. L. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod. Toxicol. 9, 233-238 (1995).
- Pelch, K. E. Aberrant gene expression profile in a mouse model of endometriosis mirrors that observed in women. Fertil. Steril. 93, 1615-1627 (2010).
- Flores, I. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil. Steril. 87, 1180-1199 (2007).
- Giudice, L. C., Kao, L. C. Endometriosis. Lancet. 364, 1789-1799 (2004).
- Lin, Y. J., Lai, L. ei, Y, H., Wing, L. Y. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 147, 1278-1286 (2006).
- Fang, Z. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil. Steril. 82, 673-678 (2004).
- Fang, Z. Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. J. Clin. Endocrinol. Metab. 87, 3460-3466 (2002).
- Cummings, A. M., Metcalf, J. L., Birnbaum, L. Promotion of endometriosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats and mice: time-dose dependence and species comparison. Toxicol. Appl. Pharmacol. 138, 131-139 (1996).
- Foster, W. G. Morphologic characteristics of endometriosis in the mouse model: application to toxicology. Can. J. Physiol. Pharmacol. 75, 1188-1196 (1997).
- Cummings, A. M., Metcalf, J. L. Effect of surgically induced endometriosis on pregnancy and effect of pregnancy and lactation on endometriosis in mice. Proc. Soc. Exp. Biol. Med. 212, 332-337 (1996).
- Lu, Y., Nie, J., Liu, X., Zheng, Y., Guo, S. W. Trichostatin A, a histone deacetylase inhibitor, reduces lesion growth and hyperalgesia in experimentally induced endometriosis in mice. Hum. Reprod. 25, 1014-1025 (2010).
- Whitten, W. K. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J. Endocrinol. 13, 399-404 (1956).
- Whitten, W. K., Bronson, F. H., Greenstein, J. A. Estrus-inducing pheromone of male mice: transport by movement of air. Science. 161, 584-585 (1968).
- Goldman, J. M., Murr, A. S., Cooper, R. L. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth. Defects. Res. B. Dev. Reprod. Toxicol. 80, 84-97 (2007).
- Pritchett-Corning, K. R., Mulder, G. B., Luo, Y., White, W. J. Principles of Rodent Surgery for the New Surgeon. J. Vis. Exp. (47), e2586-e2586 (2011).
- Moen, M. H., Halvorsen, T. B. Histologic confirmation of endometriosis in different peritoneal lesions. Acta. Obstet. Gynecol. Scand. 71, 337-342 (1992).
- Cummings, A. M. Methoxychlor as a model for environmental estrogens. Crit. Rev. Toxicol. 27, 367-379 (1997).
- Fowler, R. E., Edwards, R. G. Induction of superovulation and pregnancy in mature mice by gonadotrophins. J. Endocrinol. 15, 374-384 (1957).
- Wilson, E. D., Zarrow, M. X. Comparison of superovulation in the immature mouse and rat. J. Reprod. Fertil. 3, 148-158 (1962).
- Lee, B., Du, H., Taylor, H. S. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol. Reprod. 80, 79-85 (2009).
- Somigliana, E. Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum. Reprod. 14, 2944-2950 (1999).
- Hirata, T. Development of an experimental model of endometriosis using mice that ubiquitously express green fluorescent protein. Hum. Reprod. 20, 2092-2096 (2005).
- Story, L., Kennedy, S. Animal studies in endometriosis: a review. Ilar. J. 45, 132-138 (2004).
- Cummings, A. M., Hedge, J. M., Birnbaum, L. S. Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol. Sci. 52, 45-49 (1999).
- Cummings, A. M., Metcalf, J. L. Effects of estrogen, progesterone, and methoxychlor on surgically induced endometriosis in rats. Fundam. Appl. Toxicol. 27, 287-290 (1995).
- Sharpe-Timms, K. L. Endometriotic lesions synthesize and secrete a haptoglobin-like protein. Biol. Reprod. 58, 988-994 (1998).
- Yavuz, E., Oktem, M., Esinler, I., Toru, S. A., Zeyneloglu, H. B. Genistein causes regression of endometriotic implants in the rat model. Fertil. Steril. 88, 1129-1134 (2007).
- Dmitrieva, N. Endocannabinoid involvement in endometriosis. Pain. 151, 703-710 (2010).
- Efstathiou, J. A. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil. Steril. 83, 171-181 (2005).
- Becker, C. M. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil. Steril. 84, Suppl 2. 1144-1155 (2005).
- Becker, C. M. Short synthetic endostatin peptides inhibit endothelial migration in vitro and endometriosis in a mouse model. Fertil. Steril. 85, 71-77 (2006).
- Sharpe-Timms, K. L. Using rats as a research model for the study of endometriosis. Ann. N.Y. Acad. Sci. 955, 318-327 (2002).
- Stilley, J. A., Woods-Marshall, R., Sutovsky, M., Sutovsky, P., Sharpe-Timms, K. L. Reduced Fecundity in Female Rats with Surgically Induced Endometriosis and in Their Daughters: A Potential Role for Tissue Inhibitors of Metalloproteinase 1. Biol. Reprod. 80, (2009).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone