Method Article
Detection and Isolation of Circulating Melanoma Cells using Photoacoustic Flowmetry
W tym Artykule
Podsumowanie
We have developed a flow cytometer using laser induced ultrasound to detect circulating melanoma cells as an early indicator of metastatic disease.
Streszczenie
Circulating tumor cells (CTCs) are those cells that have separated from a macroscopic tumor and spread through the blood and lymph systems to seed secondary tumors1,2,3. CTCs are indicators of metastatic disease and their detection in blood samples may be used to diagnose cancer and monitor a patient′s response to therapy. Since CTCs are rare, comprising about one tumor cell among billions of normal blood cells in advanced cancer patients, their detection and enumeration is a difficult task. We exploit the presence of pigment in most melanoma cells to generate photoacoustic, or laser induced ultrasonic waves in a custom flow cytometer for detection of circulating melanoma cells (CMCs)4,5. This process entails separating a whole blood sample using centrifugation and obtaining the white blood cell layer. If present in whole blood, CMCs will separate with the white blood cells due to similar density. These cells are resuspended in phosphate buffered saline (PBS) and introduced into the flowmeter. Rather than a continuous flow of the blood cell suspension, we induced two phase flow in order to capture these cells for further study. In two phase flow, two immiscible liquids in a microfluidic system meet at a junction and form alternating slugs of liquid6,7. PBS suspended white blood cells and air form microliter slugs that are sequentially irradiated with laser light. The addition of a surfactant to the liquid phase allows uniform slug formation and the user can create different sized slugs by altering the flow rates of the two phases. Slugs of air and slugs of PBS with white blood cells contain no light absorbers and hence, do not produce photoacoustic waves. However, slugs of white blood cells that contain even single CMCs absorb laser light and produce high frequency acoustic waves. These slugs that generate photoacoustic waves are sequestered and collected for cytochemical staining for verification of CMCs.
Protokół
1. Blood sample preparation
- Draw approximately 10 mL of whole blood from a Stage IV Cancer patient into two 5 mL vacutainers.
- Centrifuge the tubes for ten minutes at 3,000 revolutions per minute (rpm).
- The blood sample will separate into three different layers: the bottom layer comprises the erythrocytes, the middle layer, also called the buffy coat, contains leukocytes and melanoma cells, and the top layer contains plasma and platelets. Remove as much plasma as possible without disturbing the buffy coat.
- Place 250 μL of Histopaque 1077 in two 1 mL Wintrobe tubes.
- Using a 9″ Pasteur Pipette, extract the remaining plasma, the entire buffy coat, and even the top layer of erythrocytes, making sure to collect < 500 μL.
- Place the solution atop the Histopaque layer and centrifuge the Wintrobe tubes in 15 mL conical tubes for 10 minutes at 3000 rpm.
- The blood will again separate, but this time the erythrocytes will be separated by the Histopaque, allowing for easy extraction of the buffy coat. Use a 9″ Pasteur Pipette to obtain the entire buffy coat and place it in a 2 mL eppendorf tube.
- Suspend the buffy coat in 1.5 mL of PBS with 2% v/v Tween 20.
2. Flow Chamber Construction
- Obtain an acrylic cylinder, 20 mm outer diameter, 17 mm inner diameter, and 8 mm tall. Drill three 5 mm diameter holes through the side of the cylinder at 90 degree angles from each other. Cut three pieces of polymer tubing (1.6 mm inner diameter, 5 mm outer diameter), and place them into the drilled holes.
- Stretch Parafilm across the bottom of the acrylic ring to make a water tight seal. The parafilm forms a shallow bowl with the acrylic ring and will hold the acrylamide solution that will form a flow chamber.
- Suspend a 1.6 mm diameter wire through the pieces of tubing that are across from each other, making sure that the wire alone is visible through the middle of the ring. Fill the third piece of tubing with a 1.6 mm diameter wire and position the tube 1 mm away from the suspended wire. These wires prevent acrylamide from entering the tubing. After the acrylamide solidifies, the wires will be extracted, leaving a cylindrical flow path and a place to position an optical fiber focuses onto the flow path.
- Add 5 mL of prepared acrylamide [20 g acrylamide (Sigma Aldrich), 0.7 g bisacrylamide (Sigma Aldrich), 100 mL of distilled water] to a small beaker. Measure out 0.02 g of ammonium persulfate (Sigma) and add it to the beaker. Mix with a stir bar for 3 minutes. Add 20 μL tetramethylethylenediamene (Fisher Scientific) to the beaker. Quickly pour the mixture into the acrylic ring, filling to the top. After a few seconds, the solution will gel, making a flow chamber mold. Carefully pull the wires out, making sure not to disturb the acrylamide gel. The flow chamber is now ready for use.
3. Photoacoustic flowmetry set-up
- Acoustically couple a polyvinylidene fluoride piezoelectric acoustic transducer (Panametrics) to a flow chamber using ultrasound gel directly above the flow path.
- Using a Bayonet Neill-Concelman (BNC) cable, connect the transducer to the input of a RITEC Broadband Receiver-640A (lowpass filter: out, highpass filter: 1 MHz, imput impedance: 50 Ohms, amplification: 35 dB). Connect the output of the filter to the oscilloscope (Tektronix TDS 2024B). To trigger the oscilloscope, a photodiode (Thorlabs) is set up near the transducer and is plugged into the oscilloscope using a BNC cable.
- A t-connector (Small Parts) is attached to one arm of the flow chamber. Two syringe pumps (Braintree Scientific) are connected to the other branches of the connector. One syringe contains air, while the other will contain the cell suspension prepared in part 1. Set the flow rates to 100 μL/minute.
- Extract the small tubing of the flow chamber until a small pocket of air forms, and insert a 1 mm diameter core optical fiber with a 0.37 numerical aperture in that slot. Fill the pocket with water to reduce interface signals and increase the amount of laser light applied to the flow path. Due to the numerical aperture of the optical fiber and short distance to the flow path, the laser will only irradiate one slug of sample at any given time, ensuring that the sample directly in front of the laser is the same sample creating photoacoustic signals. The laser energy should be approximately 28 mJ/cm2 with a spot size of 7 mm2.
- Turn both syringe pumps on. When the two immiscible fluids reach the t-junction they will be partitioned into homogeneous slugs and flow past the transducer.
- Irradiate the flowing sample with a nanosecond pulsed laser to generate photoacoustic waves in the melanoma cells. As melanin is a broadband optical absorber, any visible laser wavelength can be used.
- If a signal appears on the oscilloscope when a slug is atop the transducer, the slug contains melanoma, and should be tracked through the system until dropping into a collection vial.
4. Immunocytochemistry
- Take the captured slugs to a Cytospin 4 Centrifuge (Thermo Scientific) in order to fix them to glass slides. Place glass slides into Cytofunnels (Shandon EZ Single Cytofunnel with Brown Filter Card Thermo Scientific) and load them into the Cytospin 4 Centrifuge. Put 100 μL of 1% Bovine Serum Albumin (Sigma) in PBS into each funnel and then centrifuge at 600 rpm for 3 minutes to help the captured cells stick to the glass slides.
- After centrifugation, add the cell samples to the Cytofunnels and then centrifuge at 600 rpm for 3 minutes. Then spray the slides with an ethanol based fixative.
- Wash the slides in PBS for 10 minutes twice.
- To block nonspecific binding, incubate each slide with a mixture of 10 μL of Goat Serum (Sigma) and 90 μL of PBS for 1 hour at room temperature.
- After the incubation, decant the slides and incubate with the primary antibody solution for 1 hour in a humidified chamber at room temperature. Each slide should contain 1% MART-1 antibody raised in rabbit, 1% CD-45 antibody raised in mouse, 10% Goat serum, and 89% PBS.
- After incubation, wash the slides in PBS for 10 minutes three times.
- The slides are then incubated in a secondary antibody solution, which has fluorophore bound to the antibodies. The mixture contains 0.05% goat anti-rabbit antibody, 0.05% goat anti-mouse antibody, 0.1% goat serum, and 99% PBS. The slides are incubated in the dark for 1 hour at room temperature.
- After incubation, decant the slides wash in PBS for 5 minutes three times.
- To stain for a nucleus, incubate the cells with 0.1 μg/mL of DAPI (Invitrogen) for 1 minute. Then decant the slides and wash in PBS.
- Mount a coverslip over the cell sample using 20 μL of a resin-based mounting media per slide. Seal the coverslip with clear nail polish to preserve the slide. Now the slides are ready to be imaged with a fluorescent microscope.
5. Representative Results:
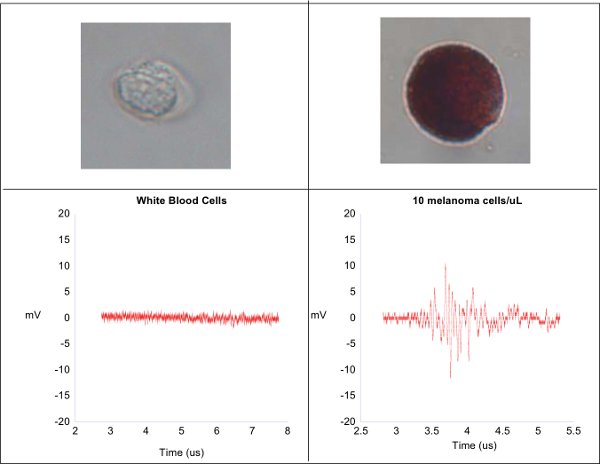
Figure 1.
The photoacoustic waveforms from white blood cells and melanoma cells are shown here. The white blood cells, having no inherent pigmentation, produce no photoacoustic waves and manifest as a flat line of electronic noise (left). The pigmented melanoma cells produces a robust photoacoustic wave (right).
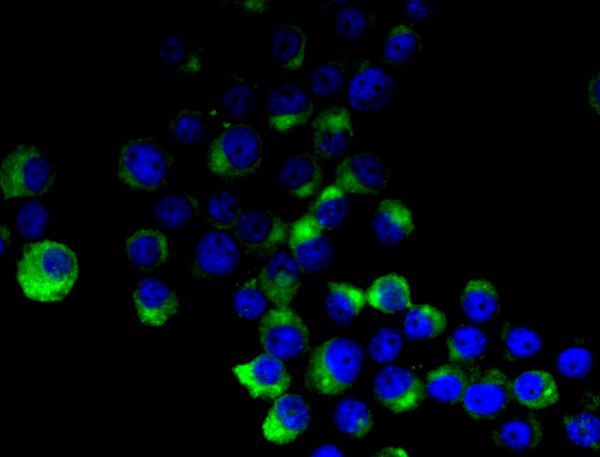
Figure 2.
After immunocytochemical staining, cultured melanoma cells show DAPI signals in blue while MART1 is highlighted in green.
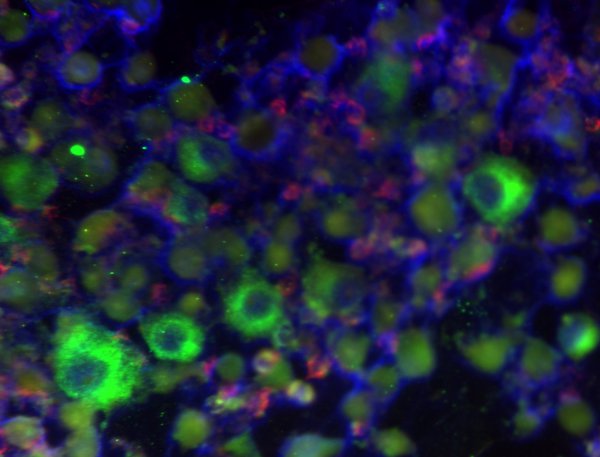
Figure 3.
Overlaying images for DAPI and MART1, as in Figure 2, with CD45 as an indicator of leukocytes, this figure shows melanoma cells among several white blood cells.
Dyskusje
Detecting CTCs is still a research intensive field with very few clinical applications due to the difficult problem of isolating the rare tumor cells. Many other techniques are being evaluated for CTC detection, including RT-PCR, microfluidic cell capture, immunomagnetic capture, and other methods8-12. However, photoacoustic detection of CMCs shows promise as it is label free and provides a rapid and accurate means to capture small, light absorbing particles.
The protocol described to isolate white blood cells is highly efficient, yet a low number of red blood cells exist in the sample. These rouge contaminating cells also absorb laser light, but at a greatly decreased level. To ensure the red blood cells do not interfere with our melanoma detection system, five healthy patient samples were introduced through the system and none gave a signal, indicating that red blood cells are present at low enough concentrations to be disregarded.
A series of concentration studies have been conducted using cultured melanoma cells and the photoacoustic flow system has proven sensitive down to 1 cell/μL (unpublished data). These studies are on going and will soon include trials using cancer patient samples.
This photoacoustic technique is also being evaluated for use in non-pigmented CTCs, such as breast and prostate cancer by attaching exogenous chromophores. We have performed preliminary work using gold nanoparticles on cancer cells13. These tests have shown that nanoparticles can provide the necessary optical absorption to generate photoacoustic waves. The remaining technical challenge is to attach such particles to cancer cells selectively.
Ujawnienia
John A. Viator is founder and president of Viator Technologies Inc, a company formed to commercialize photoacoustic technologies for the improvement of human health.
Podziękowania
We acknowledge the support of the Department of Biological Engineering and the Christopher S. Bond Life Sciences Center at the University of Missouri. We acknowledge grant support from Missouri Life Sciences Research Board 09-1034 and NIH R21CA139186-0. We also thank the Life Sciences Undergraduate Research Opportunity Program at the University of Missouri, the University of Missouri Molecular Cytology Core, and the University of Missouri College of Engineering for financial support.
Materiały
| Name | Company | Catalog Number | Comments |
| DMEM | Invitrogen | ABCD1234 |
Odniesienia
- Pantel, K., Brakenhoff, R., Brandt, B. Detection clinical relevance and specific biological properties of disseminating tumour cells. Nature Reviews Cancer. 8, 329-340 (2008).
- Pantel, K., Brakenhoff, R. Dissecting the metastatic cascade. Nature Reviews Cancer. 4, 448-456 (2004).
- Yu, M., Stott, S. Circulating tumor cells: approaches to isolation and characterization. The Journal of Cell Biology. 192, 373-373 (2011).
- Weight, R. M., Dale, P. S., Viator, J. Detection of circulating melanoma cells in human blood using photoacoustic flowmetry. , 106-109 (1985).
- Weight, R. M., Dale, P. S., Lisle, A., Caldwell, C., Viator, J. A. Photoacoustic detection of metastatic melanoma cells in the human circulatory system. Opt. Lett. 31, 2998-3000 (2006).
- Gupta, A., Kumar, R. Effect of geometry on droplet formation in the squeezing regime in a microfluidic T-junction. Microfluidics and Nanofluidics. 8, 799-812 (2010).
- Mocellin, S., Hoon, D., Ambrosi, A., Nitti, D., Rossi, C. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clinical Cancer Research. 12, 4605-4605 (2006).
- Cristofanilli, M., Budd, G., Ellis, M., Stopeck, A., Matera, J., Miller, M., Reuben, J., Doyle, G., Allard, W., Terstappen, L. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New England Journal of Medicine. 351, 781-781 (2004).
- Cristofanilli, M., Hayes, D., Budd, G., Ellis, M., Stopeck, A., Reuben, J., Doyle, G., Matera, J., Allard, W., Miller, M. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. Journal of Clinical Oncology. 23, 1420-1420 (2005).
- Ghossein, R., Scher, H., Gerald, W., Kelly, W., Curley, T., Amsterdam, A., Zhang, Z., Rosai, J. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. Journal of Clinical Oncology. 13, 1195-1195 (1995).
- Loberg, R., Fridman, Y., Pienta, B., Keller, E., McCauley, L., Taichman, R., Pienta, K. Detection and isolation of circulating tumor cells in urologic cancers: a review. Neoplasia. 6, 302-302 (2004).
- Viator, J. A., Gupta, S. K., Goldschmidt, B. S., Bhattacharyya, K., Raghuraman, R., Shukla, R., Dale, P. S., Boote, E., Katti, K. Detection of gold nanoparticle enhanced prostate cancer cells using photoacoustic flowmetry with optical reflectance. J. Biomed. Nanotech. 6, 187-191 (2010).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone