Method Article
Em Gene Vivo Transferência de células de Schwann no nervo ciático de roedores por Eletroporação
Neste Artigo
Resumo
Here, we present an in vivo technique for gene transfer to Schwann cells (SCs) in the rodent sciatic nerve. This simple technique is useful for investigating signaling mechanisms involved in the development and maintenance of myelinating SCs.
Resumo
The formation of the myelin sheath by Schwann cells (SCs) is essential for rapid conduction of nerve impulses along axons in the peripheral nervous system. SC-selective genetic manipulation in living animals is a powerful technique for studying the molecular and cellular mechanisms of SC myelination and demyelination in vivo. While knockout/knockin and transgenic mice are powerful tools for studying SC biology, these methods are costly and time consuming. Viral vector-mediated transgene introduction into the sciatic nerve is a simpler and less laborious method. However, viral methods have limitations, such as toxicity, transgene size constraints, and infectivity restricted to certain developmental stages. Here, we describe a new method that allows selective transfection of myelinating SCs in the rodent sciatic nerve using electroporation. By applying electric pulses to the sciatic nerve at the site of plasmid DNA injection, genes of interest can be easily silenced or overexpressed in SCs in both neonatal and more mature animals. Furthermore, this in vivo electroporation method allows for highly efficient simultaneous expression of multiple transgenes. Our novel technique should enable researchers to efficiently manipulate SC gene expression, and facilitate studies on SC development and function.
Introdução
The rapid transmission of sensory and motor information in the peripheral nervous system is permitted by the myelin sheath, which is formed by myelinating Schwann cells (SCs)1. Insulation of axons by the myelin sheath enables saltatory conduction, which increases the speed of nerve impulses. In disorders in which the development or maintenance of the myelin sheath is impaired, nerve conduction speed is reduced. This results in neuropathy involving motor and sensory dysfunction. Although there are many studies on the molecular mechanisms of myelination and demyelination in the peripheral nervous system, the roles of the numerous proteins involved in these processes remain unclear.
To study the molecular mechanisms of SC myelination/demyelination in vivo, genetic approaches have been used to modify gene expression in animals. A powerful approach is the use of knockout/knockin or transgenic animals. However, the generation of these animals is expensive and time consuming. For SC-specific gene manipulation, crossing floxed strains with Cre mice or other conditional gene expression methods are necessary. This again is laborious and time intensive. In recent years, a cutting-edge genetic technology, the CRISPR-Cas9 system, has made the generation of genetically modified mice much quicker (about 4 weeks)2,3, but this method is hindered by target sequence limitations, and suffers from off-target effects. As an alternative method, viral vector-mediated gene transfer is a faster and easier method of achieving gene transfer into SCs in vivo4-6. Indeed, the generation of viral vectors is less expensive, and takes a shorter time (within a few weeks), and gene manipulation of SCs can be achieved by simply injecting engineered viral vectors, such as adenoviral vectors, adeno-associated viral (AAV) vectors, and lentiviral vectors, into the sciatic nerve. Because these viral vectors have different characteristics, users have to choose the one best suited for their purpose. Adenoviral vectors infect axons and SCs in both young and mature sciatic nerves. In particular, adenoviral vectors have higher selectively for non-myelinating SCs than myelinating SCs. Adenoviruses can cause immune responses, and accordingly, immunodeficient strains should be used5. AAV vectors are currently the most widely used viral vectors, and allow in vivo gene transfer with lower toxicity7. AAV can transduce both axons and SCs by direct injection into the nerve fibers8,9. However, AAV-mediated protein expression usually requires 3 weeks or longer to reach maximum levels7,9. Therefore, it is difficult to analyze myelination, which actively progresses during the two week postnatal period. Lentiviral vectors have higher selectively for myelinating SCs than non-myelinating SCs, and do not have toxic effects on sciatic nerves. However, lentiviral vectors do not infect SCs in more mature nerves5, and therefore are unsuitable for analyzing events such as the demyelination process.
Electroporation is another faster and easier approach to achieve in vivo gene transfer. It has been reported that in vivo transfection of SCs can be achieved when electroporation is applied to transected rat sciatic nerves10. However, because this method requires nerve transection for gene delivery, the application is limited to the analysis of the damaged nerves. Here, we describe an alternative method that allows the delivery of transgenes into myelinating SCs in intact rat sciatic nerves using electroporation11. This method requires plasmid construction, which can usually be completed within a week. Then, by simply delivering electric pulses to the site on the sciatic nerve where the plasmid DNA was injected, highly selective transfection of myelinating SCs can be achieved in neonatal as well as in more mature animals. By electroporating multiple plasmids, simultaneous expression of a variety of genes can be easily achieved. The ability to simultaneous express multiple molecules, such as signaling proteins, short-hairpin RNAs (shRNAs) and functional probes, is crucial for investigating complex processes such as myelination and demyelination. The novel in vivo electroporation method described in this paper will be a powerful tool, allowing researchers to analyze the function of a multitude of molecules and their interactions in myelinating SCs.
Protocolo
O uso de ratos para a investigação estava em conformidade com as orientações estabelecidas pelo Comitê da Universidade de Tóquio Animal Welfare.
1.Preparation de DNA de plasmídeo
- Gerar os plasmídeos de ADN para electroporação in vivo por subclonagem do ADNc ou sequência de shRNA em um plasmídeo de expressão para células de mamífero 12. Usar um citomegalovírus precoce imediato intensificadoras e de frango β-actina promotor de fusão (CAG) plasmídeo conduzido pelo promotor 13 porque permite a expressão forte e estável. Para a expressão de shRNAs sob o controlo de um promotor CAG, usar um sistema de cassete shRNA baseada mir30 para subclonagem a 14 shRNA.
- Purifica-se DNA de plasmídeo com um kit Maxi-prep de acordo com as instruções do fabricante, e ressuspender o ADN com solução salina tamponada com HEPES (NaCl 140 mM, 0,75 mM de Na 2 HPO 4, 25 mM de HEPES, pH 7,40). Ajustar a concentração de ADN para ≥4 ug / uL.
- Preparar a solução de DNA de plasmídeo a uma concentração de 4 mg / mL, e adicionar uma quantidade mínima de corante verde rápido (concentração final de 0,01%) para marcar o local da injecção. Quando é necessária a electroporação simultânea de vários plasmídeos, ajustar a concentração total da solução de DNA de plasmídeo a 4 mg / mL.
Nota: A composição óptima dos DNAs de plasmídeo deve ser determinado de acordo com a eficiência de transfecção de cada plasmídeo.
2. A esterilização de instrumentos cirúrgicos e Saline
- instrumentos cirúrgicos autoclave e a solução de NaCl a 0,9%.
3. Preparação da micropipeta de vidro
- Puxe pipetas de vidro usando um extrator pipeta. Cortar a ponta da pipeta para um diâmetro de 30-50 ^ m. Utilize os seguintes parâmetros: calor, 600; Velocidade, 50; Tempo, 75.
4. Cirurgia animal, Injecção de ADN e electroporação
Nota: Um excessoFace a esta etapa é descrita na Figura 1. Embora o procedimento de crias de ratos são descritos aqui, o método também é aplicável a animais mais maduros, utilizando o mesmo procedimento.
- Anestesiar ratos com isoflurano na caixa de indução até que o animal torna-se imóvel, ajustando o fluxo de oxigénio para 0,4 L / min e a concentração de isoflurano a 4% (vol / vol). Execute toe beliscar para confirmar anesthetization adequada.
- Colocar o rato sobre o aquecedor pré-aquecido sob um microscópio binocular e manter a anestesia por administração de isoflurano continuamente através da máscara facial. Ajustar o fluxo de oxigénio para 0,2 L / min e a concentração de isoflurano a 2% (vol / vol). Use colírios para evitar a secura dos olhos, se os olhos do animal estão abertas.
- Fixar as pernas com fita cirúrgica.
- Limpar a pele na parte posterior da coxa com povidona-iodo, e fazer uma incisão com um bisturi.
Nota: Raspar áreas cirúrgicas se as áreas cirúrgicas são cobertas com har. - Expor o nervo ciático, criando uma abertura entre os músculo quadríceps femoral e músculo bíceps femoral com agulhas de costura.
- Molhar o nervo com uma solução de NaCl a 0,9%. Absorver o excesso de água com papel sem fiapos.
- Inserir a base de uma micropipeta de vidro na tubagem flexível, e preencher a quantidade adequada de solução de ADN (pelo menos um microlitro) na micropipeta, aspirando suavemente.
- Levantar o nervo exposto puxando suavemente o lado distal do nervo usando uma agulha.
Nota: Não aplicar tensão no nervo para minimizar o stress mecânico. - Inserir a micropipeta de vidro no local distai do nervo, e injectar a solução de DNA através da aplicação de pressão (isto é, por sopro para dentro da extremidade aberta do tubo flexível). Injectar a solução de DNA até o nervo aparece verde (máximo de 1 ml). Porque inserção frequente da micropipeta pode danificar o nervo, não insira o micropipeta mais de duas vezes.
- Coloque uma TWEezer tipo eletrodo de platina cerca de 1-2 mm do nervo. Preencher a lacuna entre o eletrodo e o nervo com uma solução de NaCl a 0,9%.
Nota: Não segure o nervo com o eletrodo para evitar estresse mecânico sobre o nervo. - Aplicar impulsos eléctricos para o local da injecção usando um electroporador com o eléctrodo. Após o primeiro conjunto de pulso, inverter o eletrodo e aplicar outro conjunto de pulso. Utilize os seguintes parâmetros: tensão, 50 V; duração do pulso, 5 ms; intervalo de pulso, a 100 ms; número de pulsos, 4 vezes.
- Limpar o local de electroporação com uma solução de NaCl a 0,9%.
- Repita os passos 4,4-4,11 sobre o nervo ciático contralateral.
5. Pós-eletroporação
- Fechar as incisões com cola de cianoacrilato.
- Após a secagem da cola, limpar a ferida com povidona-iodo.
- Solte o filhote de cachorro da máscara facial. Aqueça o filhote em um mais quente, pelo menos durante uma hora, a fim de permitir que ele se recuperar totalmente da anestesia. Do não deixar o cachorro sem supervisão até que tenha recuperado a consciência suficiente.
- Após a recuperação da anestesia, devolver o cachorro para o rato mãe. Não devolva o filhote até que esteja totalmente recuperado.
6. Pós-cirurgia
- Casa os filhotes de ratos na gaiola até a realização dos experimentos 11 (ver exemplos na Figura 3). Administrar carprofeno (5 mg / kg; ip), uma droga não-esteróide anti-inflamatório, ou buprenorfina (0,1 mg / kg, sc), um analgésico opióide, se necessário.
Nota: Se o filhote de rato não cresce bem ou inflamação é observado ao redor do local da cirurgia, excluir o animal a partir dos experimentos.
Resultados
Um exemplo de um nervo ciático transfectada com proteína fluorescente vermelha (RFP) -expressing plasmídeo é mostrada na Figura 2A. As células que mostram morfologia bipolar, uma característica de SCs, foram escassamente transfectadas com RFP. Nenhuma fluorescência RFP foi detectado em axónios. Normalmente encontramos ~ 100 SCs transfectadas em cada nervo. Esta eficiência de transfecção parece semelhante à in vivo SC eficiência de infecção utilizando vectores lentivirais 4.
Experimentos imunocoloração mostrou que a maioria (~ 96%) as células RFP-positivo em P7 co-marcadas durante S100, um marcador SC (Figura 2B), e 91% de células RFP-positivo em P14 co-marcadas durante MBP, um marcador mielinizantes SC (Figura 2C), o que sugere que a transferência genética através de electroporação é altamente selectivo para myelinating SCs.
A introdução de genes em múltiplas SCs in vivo será extremamente útil para investigar os mecanismos de mielinização / desmielinização. Uma grande vantagem do método de electroporação in vivo descrito aqui é a capacidade de transferência de genes múltiplos com um procedimento simples. A Figura 2D mostra uma imagem representativa de um nervo ciático transfectadas com uma mistura de GFP e os plasmídeos que expressam RFP utilizando electroporação em vivo. Cerca de 97% das SC foram GFP e RFP duplo positivo, sugerindo que a entrega altamente eficiente de genes múltiplos pode ser conseguido simplesmente electroporating misturas de vários plasmídeos.
Em roedores, a mielinização inicia em torno do nascimento, aumentando dramaticamente durante as primeiras duas semanas após o parto, e depois diminui gradualmente. Assim, manipulando geneticamente SCs durante essas janelas de tempo de desenvolvimento, os mecanismos subjacentes a estas diferentes fases de mielinização pode ser esclarecida. lentivirais são uma boa ferramenta para umnalyzing mielinização, especialmente como eles têm uma toxicidade mínima, mas lentivírus única infectar neonatal nervo ciático 5,6. Em comparação, a transferência de genes mediada por electroporação funciona bem quando a transfecção é realizada na P3 (Figura 2E, topo) ou em P14 (Figura 2E, parte inferior).
As aplicações do novo método em electroporação in vivo são descritos aqui. A Figura 3A mostra que as imagens ao microscópio óptico de GFP-expressando myelinating SCs em várias fases do desenvolvimento (P7, P14, P21 e P31). Por análise microscópica de luz, alterações nos parâmetros morfológicos, tais como comprimento e diâmetro, pode ser avaliado. Note-se que estes parâmetros têm valores semelhantes em comparação com ratos intactos 15,16 nervos periféricos, o que sugere que os nervos electroporadas desenvolver, sem efeitos prejudiciais significativos. A Figura 3B mostra uma imagem microscópica de electrões de LacZ-expressing myelinating SCs. Neste caso, LacZ foi utilizada como um marcador de expressão. β-galactosidase coloração utilizando Bluo-gal, um substrato insolúvel em etanol, possibilita a análise da estrutura de mielina de SCs transfectadas por microscopia eletrônica 11,17. Nestas experiências, o papel de moléculas de sinalização pode ser examinado por silenciamento ou aumentando a sua expressão, permitindo assim a análise da perda de função ou efeitos de ganho de função. Para além da análise de tecido fixo, in vivo, transferência de genes mediada por electroporação pode ser também aplicado a viver experiências de imagiologia. Por exemplo, a Figura 3C mostra uma mielinizantes SC co-expressando G-GECO1.1 18, um indicador verde fluorescente citosólica de Ca2 +, e R-GECO1mt 19, um indicador vermelho fluorescente Ca2 + mitocondrial. Ao expressar estes indicadores, identificamos uma via de sinalização que controla citosólica e mitocondrial de Ca2 + concentrações em myelinating SCs . Assim, o presente método pode ser usado para estudar uma variedade de mecanismos de sinalização, especialmente quando as sondas fluorescentes geneticamente codificados estão disponíveis para detectar os sinais de interesse.
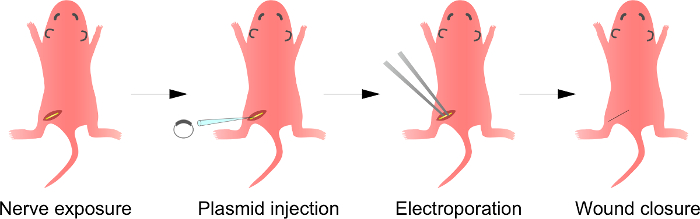
Figura 1:. Esquemática do i n vivo A electroporação Método Em primeiro lugar, o nervo ciático do rato anestesiado é exposta. Em segundo lugar, o ADN de plasmídeo é injectado no nervo ciático. Terceiro, os impulsos eléctricos são entregues no local da injecção, através do eléctrodo de uma pinça em forma de. Finalmente, a ferida é fechada com cola. Este procedimento pode ser repetido sobre o nervo contralateral. Por favor clique aqui para ver uma versão maior desta figura.
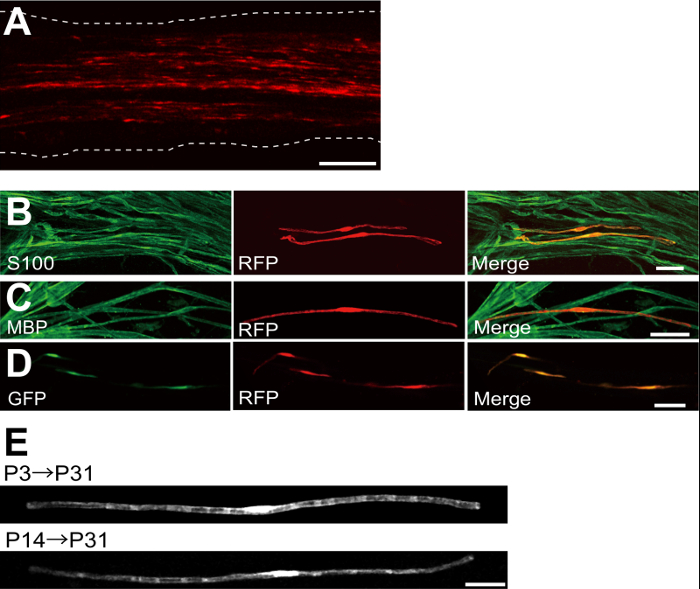
Figura 2: Resultados representativos sobre nervos ciáticos transfectadas (A) uma imagem representativa de um nervo ciático transfectadas.. O nervo foi transfectada com o plasmídeo que expressa RFP no P3, e fixado em P7. (B) uma imagem representativa de uma célula transfectada-RFP em P7 mostrando uma co-localização com S100, um marcador SC. (C) uma imagem representativa de um nervo ciático transfectada-RFP no P14 mostrando uma co-localização com MBP, um marcador mielinizantes SC. (D) A imagem representativa de um nervo ciático cotransfectadas com GFP e plasmídeos que expressam RFP. SCs transfectadas expressaram simultaneamente GFP e RFP. (E) Uma imagem de myelinating SCs no P31 transfectadas em P3, quando mielinização começa (em cima), e uma imagem de SCs myelinating no P31 transfectadas a P14, quando a maioria das grandes axônios se tornar mielinizado (parte inferior), sugerindo que a transfec ç ã o das SC myelinating pode ser conseguida não só em nervos neonatal, mas também em nervos mais maduros. Barras de escala = 200 uM (A); 50 mm (BE). Este valor foi modificado a partir de nossa publicação anterior 11. Por favor clique aqui para ver uma versão maior desta figura.
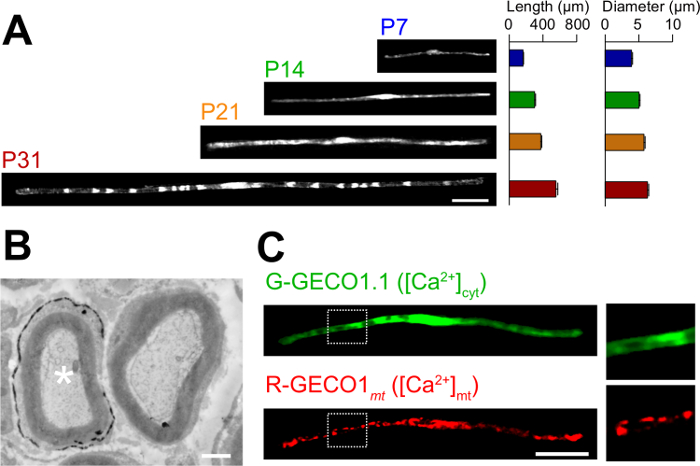
Figura 3: Aplicação de electroporação in vivo (a) uma análise microscópica de luz do desenvolvimento de myelinating SCs.. Os nervos ciáticos foram electroporadas com o plasmídeo que expressa GFP em P3, e foram fixados em vários estágios de desenvolvimento (P7, P14, P21 e P31). Imagens representativas de CT-GFP positivo estão apresentados à esquerda. A duração média e o diâmetro estão resumidos como média ± EPM (n = 30-47 de 3 nervos) no lado direito. O comprimento e diâmetro do myelinating SCs aumenta à medida que prossegue o desenvolvimento. (B) uma imagem de microscopia electrónica de um nervo ciático transfectadas com um plasmídeo que codifica para LacZ. Um transf SC (asterisco branco, à esquerda) foi finamente marcado com precipitados do produto da reacção de β-galactosidase. (C) Uma imagem de um SC cotransfectadas com G-GECO1.1, um indicador verde fluorescente citosólica de Ca2 +, e R-GECO1mt, um indicador vermelho fluorescente mitocondrial de Ca2 +. As regiões dentro dos retângulos pontilhadas brancas são mostrados ampliada nos painéis à direita. Barras de escala = 50 uM (A e C); 1 | iM (B). Este valor foi modificado a partir de nossa publicação anterior 11. Por favor clique aqui para ver uma versão maior desta figura.
Discussão
In this paper, we describe a simple and efficient method that allows in vivo gene transfer to myelinating SCs in the rat sciatic nerve using electroporation. This method allows highly selective gene expression in myelinating SCs by simply applying electric pulses to the plasmid DNA-injected sciatic nerve. Because the molecular mechanisms of myelination and demyelination in the peripheral nervous system remain unclear, the present in vivo electroporation method will be a powerful tool to clarify the roles of multiple genes of interest in living animals.
A critical requirement of this method is to keep damage to the nerve during surgery to a minimal level. Should surgical damage cause excessive inflammation, the sciatic nerve may degenerate. To avoid this, one must conduct surgery with extreme care, so as to not damage the blood vessels around the nerve. Mechanical stress to the nerve during the surgery can also be a cause of nerve damage. To minimize mechanical stress, lifting the exposed nerve should be done as gently as possible, and the tweezer-type electrode should be placed close to the nerve without contact. Furthermore, electrical pulses that are too strong can cause undesirable large leg movement, which leads to mechanical stress, or can burn the nerve. If significant damages are observed in the nerves, we recommend reducing the electrical pulse intensities or placing the electrode further away from the nerve.
In our present protocol, CAG promoter-driven plasmids were used as expression vectors. CAG promoter-driven plasmids allow high levels of gene expression in myelinating SCs in vivo. We also have tried a CMV promoter, another widely used universal promoter for mammalian gene expression, but expression of the gene product was very weak. This is consistent with previous results, in which electroporation-mediated transfection was conducted in the embryonic brain20. Therefore, we recommend using CAG promoter-driven plasmids for the in vivo electroporation method.
Because axonal signaling is a key factor in myelination/demyelination21, gene modification in neurons is also important. However, delivery of transgenes using our in vivo electroporation method is limited to SCs. It has been reported that gene delivery into sciatic nerve axons can be achieved when in vivo electroporation is applied to dorsal root ganglion (DRG) neurons in adult rats22. This suggests that delivery of plasmid DNA to the cell body is likely to be critical for in vivo transfection of peripheral axons. Thus, to examine the involvement of axonal molecules in myelination/demyelination, researchers should use neuron-specific genetic methods such as genetically modified animals, neuron-specific viral vectors, or in vivo electroporation to DRG neurons.
Compared with current methods, such as the generation of genetically modified animal lines23 and delivery of transgenes by viral vectors4-6, gene modification of SCs by in vivo electroporation is simpler. This method only requires several days for plasmid DNA construction and one day for electroporation surgery. Plasmid DNA construction does not require a biohazard room that is usually essential for viral vector handling. In addition, one of the advantages of the electroporation method is the capacity for simultaneous expression of multiple gene products using a simple protocol. Our novel technique will be useful for analyzing the interaction of a variety of signaling molecules involved in myelination and demyelination. In particular, by permitting the cotransfection of a number of different intracellular fluorescent probes, our method should be a powerful tool for investigating intracellular signaling dynamics in SCs using live imaging experiments.
Divulgações
The authors declare that they have no competing financial interests.
Agradecimentos
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology to M.I. (21229004 and 25221304).
Materiais
| Name | Company | Catalog Number | Comments |
| Genopure Plasmid Maxi Kit | Roche | 03 143 422 001 | Plasmid DNA purification kit |
| Fast Green CFC | WAKO | 069-00032 | Dye for DNA injection |
| GC 150T-10 | HARVARD APPARATUS | 30-0062 | Glass capillary |
| Suction tubing | Drummond | 05-2000-00 | Suction tubing for micro injection |
| MODEL P-97 | SUTTER INSTRUMENT CO. | Micropipette puller | |
| CUY21 Single Cell | BEX | Electroporator CUY21 Single Cell | Pulse generator |
| Electric warmer | KODEN | CAH-6A | Warmer during the surgery |
| Isofluolane | Mylan | 1119701G1076 | Anesthetic |
Referências
- Nave, K. A., Werner, H. B. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 30, 503-533 (2014).
- Wang, H., et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 153, 910-918 (2013).
- Yang, H., Wang, H., Jaenisch, R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat Protoc. 9, 1956-1968 (2014).
- Cotter, L., et al. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science. 328, 1415-1418 (2010).
- Gonzalez, S., Fernando, R. N., Perrin-Tricaud, C., Tricaud, N. In vivo introduction of transgenes into mouse sciatic nerve cells in situ using viral vectors. Nat Protoc. 9, 1160-1169 (2014).
- Ozcelik, M., et al. Pals1 is a major regulator of the epithelial-like polarization and the extension of the myelin sheath in peripheral nerves. J Neurosci. 30, 4120-4131 (2010).
- Daya, S., Berns, K. I. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 21, 583-593 (2008).
- Glatzel, M., et al. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci U S A. 97, 442-447 (2000).
- Homs, J., et al. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene Ther. 18, 622-630 (2011).
- Aspalter, M., et al. Modification of Schwann cell gene expression by electroporation in vivo. J Neurosci Methods. 176, 96-103 (2009).
- Ino, D., et al. Neuronal Regulation of Schwann Cell Mitochondrial Ca(2+) Signaling during Myelination. Cell Rep. 12, 1951-1959 (2015).
- Struhl, K. Chapter 3; Subcloning of DNA fragments. Curr Protoc Mol Biol. , Unit3 16 (2001).
- Niwa, H., Yamamura, K., Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 108, 193-199 (1991).
- Chang, K., Elledge, S. J., Hannon, G. J. Lessons from Nature: microRNA-based shRNA libraries. Nat Methods. 3, 707-714 (2006).
- Schlaepfer, W. W., Myers, F. K. Relationship of myelin internode elongation and growth in the rat sural nerve. J Comp Neurol. 147, 255-266 (1973).
- Webster, H. D. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J Cell Biol. 48, 348-367 (1971).
- Weis, J., Fine, S. M., David, C., Savarirayan, S., Sanes, J. R. Integration site-dependent expression of a transgene reveals specialized features of cells associated with neuromuscular junctions. J Cell Biol. 113, 1385-1397 (1991).
- Zhao, Y., et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science. 333 (2), 1888-1891 (2011).
- Suzuki, J., et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun. 5, 4153 (2014).
- Tabata, H., Nakajima, K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ. 50, 507-511 (2008).
- Taveggia, C., Feltri, M. L., Wrabetz, L. Signals to promote myelin formation and repair. Nat Rev Neurol. 6, 276-287 (2010).
- Saijilafu, E. M., Hur, F. Q., Zhou, Genetic dissection of axon regeneration via in vivo electroporation of adult mouse sensory neurons. Nat Commun. 2, 543 (2011).
- Tanaka, Y., Hirokawa, N. Mouse models of Charcot-Marie-Tooth disease. Trends Genet. 18, S39-S44 (2002).
Reimpressões e Permissões
Solicitar permissão para reutilizar o texto ou figuras deste artigo JoVE
Solicitar PermissãoThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados