Liquid Phase Reactor: Sucrose Inversion
Source: Kerry M. Dooley and Michael G. Benton, Department of Chemical Engineering, Louisiana State University, Baton Rouge, LA
Both batch and continuous flow reactors are used in catalytic reactions. Packed beds, which use solid catalysts and a continuous flow, are the most common configuration. In the absence of an extensive recycle stream, such packed bed reactors are typically modeled as "plug flow". The other most common continuous reactor is a stirred tank, which is assumed to be perfectly mixed.1 One reason for the prevalence of packed bed reactors is that, unlike most stirred tank designs, a large wall area to reactor volume ratio promotes more rapid heat transfer. For almost all reactors, heat must either be added or withdrawn to control the temperature for the desired reaction to take place.
The kinetics of catalytic reactions are often more complex than the simple 1st order, 2nd order, etc. kinetics found in textbooks. The reaction rates can also be affected by rates of mass transfer - reaction cannot take place faster than the rate at which reactants are supplied to the surface or the rate at which products are removed - and heat transfer. For these reasons, experimentation is almost always necessary to determine the reaction kinetics prior to designing large-scale equipment. In this experiment, we explore how to conduct such experiments and how to interpret them by finding a reaction rate expression and an apparent rate constant.
This experiment explores the use of a packed bed reactor to determine the kinetics of sucrose inversion. This reaction is typical of those characterized by a solid catalyst with liquid phase reactants and products.
sucrose → glucose (dextrose) + fructose(1)
A packed bed reactor will be operated at different flow rates to control the space time, which is related to residence time and is analogous to elapsed time in a batch reactor. The catalyst, a solid acid, will first be prepped by exchanging protons for any other cations present. Then, the reactor will be heated to the desired temperature (isothermal operation) with the flow of reactants. When the temperature has equilibrated, product sampling will begin. The samples will be analyzed by a polarimeter, which measures optical rotation. The mixture's optical rotation can be related to the conversion of sucrose, which can then be used in standard kinetics analyses to determine the order of the reaction, with respect to the reactant sucrose, and the apparent rate constant. The effects of fluid mechanics - no axial mixing (plug flow) vs. some axial mixing (stirred tanks in series) - on the kinetics will be analyzed as well.
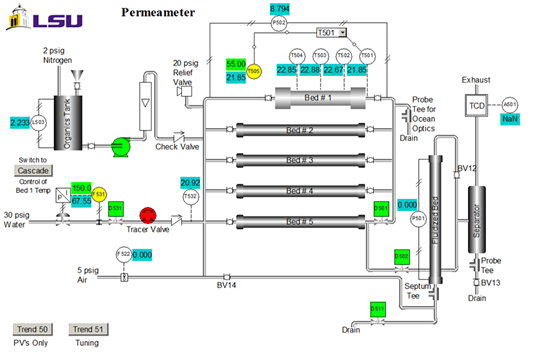
Catalyst properties are: size = 20 - 40 mesh; weight = 223 g; water content = 30 wt. %; apparent (bulk) density = 1.01 g/mL; acid site concentration = 4.6 mmol acid sites/g dry weight; surface area = 50 m2/g; macroporosity (macropore volume/total volume of cat.) = 0.34; average macropore size = 80 nm. A P&ID diagram of the unit is shown in Figure 2. For this experiment, only bed #1, the organics tank, pump and rotameter are used. Samples are collected at the upper
The polarimeter determines the fractional conversions of sucrose after reaction in a packed bed reactor. A previous polarimeter calibration for a three different sucrose feeds is shown in Figure 3.

Figure 3. Relationship between degree of rotation and fractional conversion o
The reaction does not behave exactly as expected because the apparent order n is > 1. Of all the phenomena that can cause such deviations in real reactors, deviations from ideal PFR behavior caused by axial mixing are suggested by the fact that fitting to the tanks-in-series model gives only a small number of tanks - for a perfect PFR, N should be at least 6. Such deviations are often found in relatively short beds, especially if the flow is multiphase (some water is vaporized in the reactor). However, another cause
- J. Sauer, N. Dahmen and E. Henrich. "Chemical Reactor Types." Ullman's Encycylopedia of Industrial Chemistry (2015). Web. 15 Oct. 2016.
- H.S. Fogler, "Elements of Chemical Reaction Engineering," 4th Ed., Prentice-Hall, Upper Saddle River, NJ, 2006, Ch. 2-4; O. Levenspiel, "Chemical Reaction Engineering," 3rd Ed., John Wiley, New York, 1999, Ch. 4-6; C.G. Hill, Jr. and T.W. Root, "Introduction to Chemical Engineering Kinetics and Reactor Design," 2nd Ed., John Wiley, New York, 2014, Ch. 8.
- N. Lifshutz and J. S. Dranoff, Ind. Eng. Chem. Proc. Des. Dev., 7, 266-269 (1968).
- E.R. Gilliland, H. J. Bixler, and J. E. O'Connell, Ind. Eng. Chem. Fundam., 10, 185-191 (1971).
- "Sulfuric Acid." The Essential Chemical Industry. Univ. of York, 2016. http://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html. Accessed 10/20/16.
- E. Lotero, Y. Liu, D.E. Lopez, K. Suwannakarn, D.A. Bruce and J.G. Goodwin, Jr., Ind. Eng. Chem. Res.,44, 5353-5363 (2005); A. Buasri, N. Chaiyut, V. Loryuenyong, C. Rodklum, T. Chaikwan, and N. Kumphan, Appl. Sci.2, 641-653 (2012); doi:10.3390/app2030641.
Pular para...
Vídeos desta coleção:

Now Playing
Liquid Phase Reactor: Sucrose Inversion
Chemical Engineering
9.6K Visualizações

Teste de eficiência da transferência de calor de um trocador de calor de tubos aletados
Chemical Engineering
17.8K Visualizações

Uso de um secador de bandeja para investigar a transferência de calor por convecção e condução
Chemical Engineering
43.7K Visualizações

Viscosidade de soluções de propilenoglicol
Chemical Engineering
32.1K Visualizações

Porosimetria de um pó de sílica alumina
Chemical Engineering
9.6K Visualizações

Demonstração do modelo de lei de potência por meio de extrusão
Chemical Engineering
9.9K Visualizações

Absorvedor de gás
Chemical Engineering
36.4K Visualizações

Equilíbrio vapor-líquido
Chemical Engineering
87.5K Visualizações

O efeito da taxa de refluxo na eficiência da destilação em bandejas
Chemical Engineering
77.1K Visualizações

Eficiência da Extração Líquido-Líquido
Chemical Engineering
48.1K Visualizações

Cristalização de Ácido Salicílico via Modificação Química
Chemical Engineering
24.0K Visualizações

Fluxo monofásico e bifásico em um reator de leito compactado
Chemical Engineering
18.8K Visualizações

Cinética da Polimerização por Adição ao Polidimetilsiloxano
Chemical Engineering
16.0K Visualizações

Reator Catalítico: Hidrogenação de etileno
Chemical Engineering
29.9K Visualizações

Avaliação a Transferência de Calor de um Equipamento de Resfriamento e Agitação
Chemical Engineering
7.3K Visualizações
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados