Для просмотра этого контента требуется подписка на Jove Войдите в систему или начните бесплатную пробную версию.
Method Article
Синтез индоксил-гликозидов для обнаружения гликозидазой деятельности
В этой статье
Резюме
Indoxyl glycosides are well-established and widely used tools for enzyme screening and enzyme activity monitoring. Especially for glucose type structures previous syntheses proved to be challenging and low yielding. Our novel approach employs indoxylic acid esters as precious intermediates to yield a considerable number of indoxyl glycosides in good yields.
Аннотация
Indoxyl glycosides proved to be valuable and versatile tools for monitoring glycosidase activities. Indoxyls are released by enzymatic hydrolysis and are rapidly oxidized, for example by atmospheric oxygen, to indigo type dyes. This reaction enables fast and easy screening in vivo without isolation or purification of enzymes, as well as rapid tests on agar plates or in solution (e.g., blue-white screening, micro-wells) and is used in biochemistry, histochemistry, bacteriology and molecular biology. Unfortunately the synthesis of such substrates proved to be difficult, due to various side reactions and the low reactivity of the indoxyl hydroxyl function. Especially for glucose type structures low yields were observed. Our novel approach employs indoxylic acid ester as key intermediates. Indoxylic acid esters with varied substitution patterns were prepared on scalable pathways. Phase transfer glycosylations with those acceptors and peracetylated glycosyl halides can be performed under common conditions in high yields. Ester cleavage and subsequent mild silver mediated glycosylation yields the peracetylated indoxyl glycosides in high yields. Finally deprotection is performed according to Zemplén.
Введение
В течение долгого времени производство индиго был экономически очень важный процесс. Перед крупномасштабных химических синтезов дал дешевый доступ к индиго, предшественники были получены из природных источников с дохристианских времен. Выращивание индиго обеспечения растений (естественно индиго) в Европе стал неблагодарным в 17-м веке, как количество индиго предшественники индийской индиго (0,2-0,8%) составляет около 30 раз выше. В конце 19-го века химического синтеза индиго подавил обычный выращивание 1,2.
Indigo предшественники, происходящие в растениях включают индикана (1), Insatan A (2) и Isatan B (3) (рис 1). Все они состоят из индоксил мотива, связанного с гликозильного остатка. Расщепление гликозидной связи, например, с помощью ферментативного гидролиза, приводит к выделению индоксил (4). Сама индоксил практически бесцветны, но могут быть быстро окисляется с образованием индиго Краситель (5). Это чувствительная реакция была адаптирована в биохимии, бактериологии, гистохимии и молекулярной биологии для мониторинга активности ферментов. Скрининг активности в естественных условиях без выделения или очистки ферментов, а также экспресс-тесты на чашках с агаром или в растворе (например, бело-голубым скрининга, микро-скважин) возможно. В зависимости от остатка (например, сложные эфиры, гликозиды, сульфаты), связанного с индоксил фрагмента, пригодными субстратами в различных классов ферментов (например, эстераз, глюкозидазы, сульфатазы) были разработаны 3. В следующем внимание будет уделено формированию и применению индоксил гликозидов.
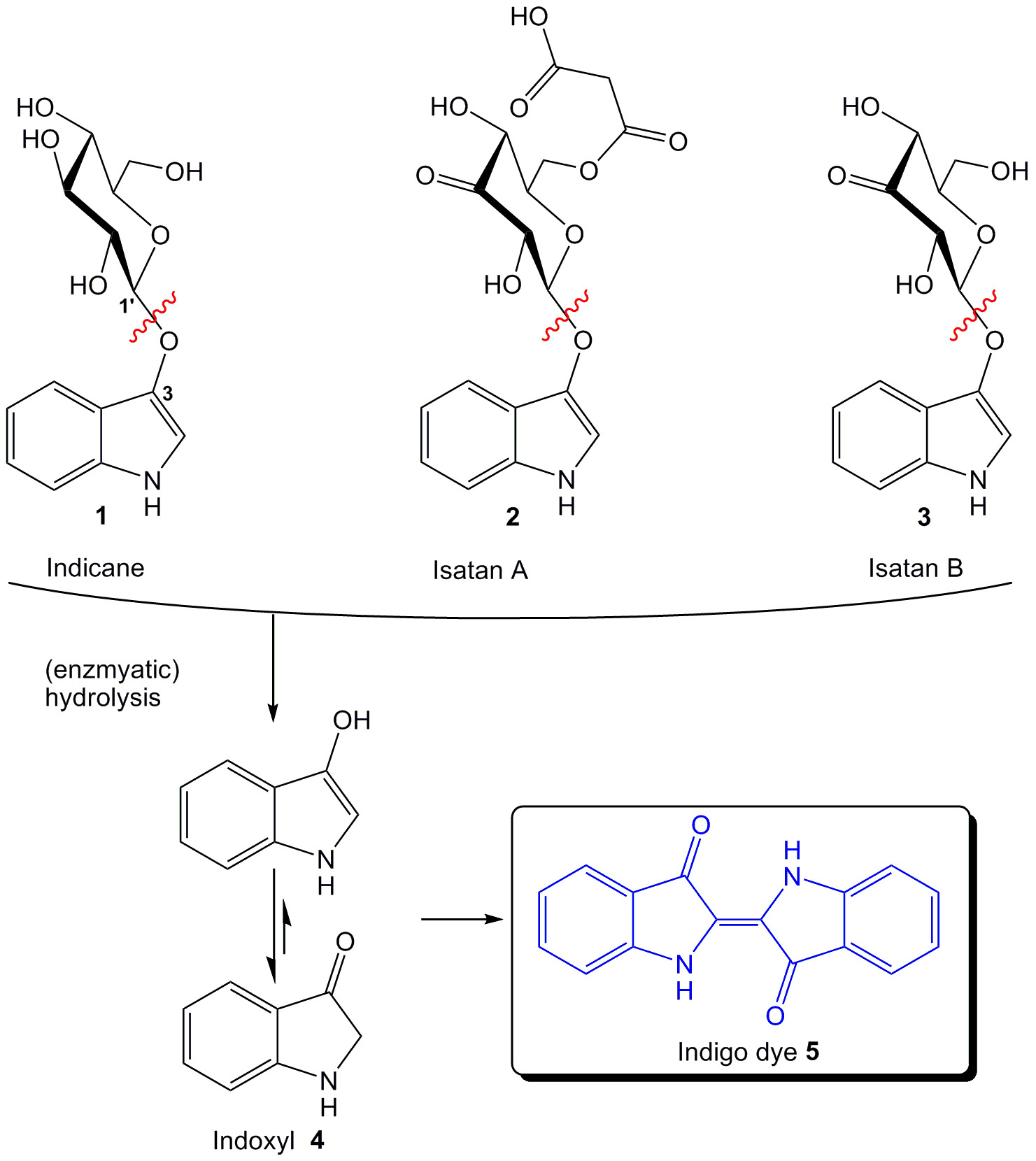
Рисунок 1: Природные предшественники индиго и формирование красителя индиго путем гидролиза.цель = "_ пустое"> Пожалуйста, нажмите здесь, чтобы посмотреть большую версию этой фигуры.
Замещение картина индоксил фрагмента определяет цвет и физические свойства полученного красителя индиго. Наиболее распространенные шаблоны замещения 5-бром-4-хлор (сокращенно от X; зеленовато-голубой), 5-бром (синий) и 5-бром-6-хлор (пурпурный), так как они образуют мельчайшие частицы красителя, делают не образуют гранулы и имеют наименьший диффузию из участков гидролиза. Последнее свойство особенно важно для естественных условиях экспериментов в 3.
Первый доклад о indigogenic метода для обнаружения эстеразы был опубликован в 1951 году и Barrnett Селигман, который занятого индоксил ацетат и бутират 4. О десятилетие спустя indigogenic принцип был адаптирован для локализации глюкозидазы млекопитающих 5. До сих пор несколько индоксил гликозидов были разработаны, хотя их синтез оказался трудно. Большинство синтезов на основе использования в -acetylated индоксил N в качестве акцептора и соответствующих гликозилгалогенидом донора 6-14. Гликозилирование проводят в ацетоне с помощью гидроксида натрия. В этих условиях количество побочных реакций происходит, значительно уменьшая выход. Специально для глюкозы структур типа очень низкие выходы гликозилирования сообщалось (например, 15% (N-ацетил-5-бром-4-хлор-индол-3-ил) -2,3,4,6-тетра-О-ацетил -β-ᴅ-глюкопиранозид 6 и 26% (N-ацетил-5-бром-4-хлор-индол-3-ил) -2,3,2 ', 3', 4'-пента вывода -acetyl- β-ᴅ-xylobioside 14 в более недавний пример). Через новый подход, используя сложные эфиры indoxylic кислоты, значительное количество индоксил гликозидов были получены с хорошим выходом (например, (N-ацетил-5-бром-4-хлор-индол-3-ил) -2,3,4, 6-тетра-О-ацетил-β-ᴅ-глюкопиранозид выход 57%).
_content "> Следующий протокол описывает простой синтез indoxylic кислоты аллилового эфира (5-бром-4-хлор) и на их основе синтеза с индоксил гликозида (X-Gal). Простой модельный эксперимент показывает ферментную реактивность β- галактозидазы с использованием X-Gal.протокол
General remarks:
All reactions were carried out using a fume hood and appropriate personal protective equipment. All reagents and solvents (p.a.; water content for dry solvents: MeCN <50 ppm; Et2O <0.01%; CH2Cl2 <0.003%; THF <0.005%) were purchased from commercial sources and used as received. TLC was performed on Merck silica gel 60 F254 plates. Compounds were detected by UV and/or by treatment with EtOH/H2SO4 (9:1) and subsequent heating. Column chromatography was performed with Merck/Fluka silica gel 60 (230-400 mesh). Filtrations were performed using Büchner funnel and aspirator pump. Solvents were removed employing a rotary evaporator (40 °C, ~200 rmp). Products were dried in high vacuum (rotary vane pump). Reactions requiring dry solvents were carried out under argon atmosphere.
In the following the synthesis of X-Gal (16) employing 5-bromo-4-chloro-indoxylic acid allyl ester (12) is described. For further compounds with varied substitution patterns as well as methyl esters prepared according to this synthetic sequence see ref.17-19.
1. Acceptor: Synthesis of 5-Bromo-4-Chloro-Indoxylic Acid Allyl Ester18 (12)
- N-Acetylation: Synthesis of N-(4-bromo-3-chloro-2-methylphenyl)-acetamide (6)
- Dissolve 10.0 g (45.7 mmol) of 4-bromo-3-chloro-2-methylaniline in 30 ml dichloromethane.
Caution! Dichloromethane is harmful and cool the solution in an ice-bath. - Add 6.50 ml (68.7 mmol) acetic anhydride dropwise and allow the mixture to warm to room temperature. The mixture can be stirred for 8 hr or overnight. Caution! Acetic anhydride is corrosive and flammable.
- Remove the solvent and recrystallize from 9:1 ethyl acetate/methanol (yield: 97% (11.6 g, 44.2 mmol), colorless solid).
Caution! Ethyl acetate is irritant and flammable. Caution! Methanol is toxic and flammable.
- Dissolve 10.0 g (45.7 mmol) of 4-bromo-3-chloro-2-methylaniline in 30 ml dichloromethane.
- Oxidation: Synthesis of N-acetyl-5-bromo-6-chloro-anthranilic acid (7)
- Reflux a mixture of 2.30 g (8.76 mmol) of 6 (product obtained from step 1.1) and 2.10 g (8.52 mmol) MgSO4•7H2O in 40 ml water in a 3-neck round bottom flask equipped with cooler and dropping funnel.
- Add dropwise 50 ml saturated KMnO4 solution in water to the reaction mixture over a period of 3 hr. Then heat for 2 additional hr under reflux, ca. 135 °C oil bath. Caution! Potassium permanganate is harmful and oxidising.
- Cool to room temperature and filter the pyrolusite (MnO2), which is formed during the reaction.
Caution! Pyrolusite is harmful. - Add hydrochloric acid (37%) to the filtrate until pH 1, then filter the product and dry in vacuum, (yield: 70% (1.80 g, 6.15 mmol), colorless solid).
Caution! Hydrochloric acid is corrosive.
- Deacetylation: Synthesis of 5-bromo-6-chloro-anthranilic acid (8)
- Dissolve 3.90 g (13.3 mmol) of 7 (product obtained from step 1.2) in 70 ml of 1 N aqueous sodium hydroxide solution and heat at ca. 100 °C (oil bath) for 5 hr.
Caution! Sodium hydroxide is corrosive. - Cool to room temperature and add hydrochloric acid (37%) until pH 1, then filter the product with a Büchner funnel and dry in vacuum, (yield: 88% (2.93 g, 11.7 mmol), colorless solid).
- Dissolve 3.90 g (13.3 mmol) of 7 (product obtained from step 1.2) in 70 ml of 1 N aqueous sodium hydroxide solution and heat at ca. 100 °C (oil bath) for 5 hr.
- Anhydride formation: Synthesis of 5-bromo-6-chloro-isatoic anhydride (9)
- Suspend 8.17 g (32.6 mmol) of 8 (product obtained from step 1.3) in 33 ml dry acetonitrile.
Caution! Acetonitrile is harmful and flammable. - Stir at room temperature and add 5.3 ml and a solution of 3.22 g (10.8 mmol) triphosgene in 18.5 ml dry dichloromethane dropwise simultaneously over a period of 30 min. Then heated for 3 hr at 50 °C (oil bath). Caution! Pyridine is harmful and flammable.
Caution! Triphosgene is toxic and can release phosgene. - Remove about 75% of the solvent and quench the reaction by adding 100 ml distilled water and filter your product. Wash with a small amount of cold dichloromethane.
- Dry the product in vacuum, (yield: 88% (7.53 g, 27.2 mmol), colorless solid).
- Suspend 8.17 g (32.6 mmol) of 8 (product obtained from step 1.3) in 33 ml dry acetonitrile.
- N-Alkylation: Synthesis of 5-bromo-6-chloro-N-[(methoxycarbonyl)allyl]-isatoic anhydride (10)
- Dissolve 25 g (90 mmol) of 9 (product obtained from step 1.4) in 250 ml anhydrous dimethylformamide. and cool with an ice-bath.
Caution! Dimethylformamide is harmful and flammable - Add 1.15 equivalents of sodium hydride (60% in paraffin) portion wise.
Caution! Sodium hydride is corrosive and flammable; heavy reaction with water/ice. - After 30 min of stirring at 0 °C allow warming to room temperature and add 1.2 equivalents allyl bromoacetate dropwise.
- After stirring for 5 hr quench the reaction by adding 500 ml distilled water and filter your product.
- Wash with water (3 times, 100 ml) and dry the product in vacuum, (yield: 93% (31.5 g, 84.1 mmol), colorless solid).
- Dissolve 25 g (90 mmol) of 9 (product obtained from step 1.4) in 250 ml anhydrous dimethylformamide. and cool with an ice-bath.
- Anhydride Opening: Synthesis of 5-bromo-6-chloro-N-(methoxycarbonylallyl)-anthranilic acid allyl ester (11)
- Dissolve 10 g (27 mmol) of 10 (product obtained from step 1.5) in 100 ml allyl alcohol.
Caution! Allyl alcohol is harmful and dangerous for the environment. - Add 350 mg (8.70 mmol) sodium hydride (60% in paraffin) portion wise.
- After stirring for 6.5 hr or overnight remove the solvent.
- Subject the crude product to column chromatography (column 30 x 6 cm; 240 g silica) petroleum ether/ethyl acetate 2:1; RF = 0.66), (yield: 80% (8.30 g, 21.3 mmol), yellow oil).
Caution! Petroleum ether is flammable, irritant and dangerous for the environment.
- Dissolve 10 g (27 mmol) of 10 (product obtained from step 1.5) in 100 ml allyl alcohol.
- Dieckmann Condensation: Synthesis of 5-bromo-4-chloro-indoxylic acid allyl ester (12)
- Mix 3.0 g (7.7 mmol) of 11(product obtained from step 1.6) and 1.75 g (15.6 mmol) of potassium tert-butoxide in 100 ml dry diethyl ether.
Caution! Potassium tert-butoxide is corrosive and flammable.
Caution! Diethyl ether is harmful and extremely flammable. - Heat the mixture under reflux (cooler) for 2 hr at 40-45 °C.
- Remove about 75% of the solvent, and add diluted hydrochloric acid (100 ml, 1 M).
Caution! Do not dry completely, decomposition can occur. - Filter the product and dry in vacuum, (yield: 84% (5.14 g, 15.5 mmol), colorless to slightly green solid).
- Mix 3.0 g (7.7 mmol) of 11(product obtained from step 1.6) and 1.75 g (15.6 mmol) of potassium tert-butoxide in 100 ml dry diethyl ether.
2. Indoxyl-Glycoside: Synthesis of X-Gal17
- Phase Transfer Glycosylation: Synthesis of (5-bromo-4-chloro-indox-3-ylic acid allyl ester) 2,3,4,6-tetra-O-acetyl-β-ᴅ-galactopyranoside (14)
- Mix 400 mg (1.21 mmol) of the indoxylic acid allylester 12 (product obtained from step 1.7), 410 mg (1.21 mmol) tetrabutylammonium hydrogensulfate and 500 mg (1.21 mmol) of the donor 2,3,4,6-tetra-O-acetyl-α-ᴅ-galactopyranosyl bromide (α-acetobromogalactose) in 10 ml dichloromethane. Caution! Tetrabutylammonium hydrogensulfate is harmful.
- Add 10 ml of an aqueous solution of potassium carbonate (1 M).
Caution! Potassium carbonate is corrosive. - Stir at room temperature until complete consumption of the donor (TLC: petroleum ether/ethyl acetate).
- Separate the organic phase (separatory funnel), dry over Na2SO4 and remove the solvent.
- Subject the crude product to column chromatography (petroleum ether/ethyl acetate 1:1; RF = 0.33). (yield: 86% (690 mg, 1.04 mmol), colorless solid).
- Ester Cleavage and Decarboxylation: Synthesis of (N-acetyl-5-bromo-4-chloro-indol-3-yl) 2,3,4,6-tetra-O-acetyl-β-ᴅ-galactopyranoside (15)
- Dissolve 600 mg (0.908 mmol) of 14 (product obtained from step 2.1) in 15 ml dry tetrahydrofurane.
Caution! Tetrahydrofurane is harmful and flammable. - Add 800 μl morpholine and 105 mg (9.09 μmol) tetrakis(triphenylphosphine)palladium(0) and stir overnight at room temperature.
Caution! Morpholine is flammable and corrosive. - Remove the solvent.
- Add 400 mg (2.40 mmol) silver acetate, 800 mg (5.79 mmol) potassium carbonate and 10 ml acetic anhydride.
Caution! Silver acetate is irritant and dangerous for the environment. - Heat at 90-100 °C for 20 min.
- After cooling to room temperature dilute with dichloromethane.
- Wash the mixture twice with water and once with a diluted NaHCO3 solution (10%).
Caution! Sodium bicarbonate is corrosive. - Dry the organic phase over Na2SO4 (filter) and remove the solvent.
- Subject the crude product to column chromatography (PE/EA 1:1; RF = 0.24), (yield: 88% (495 mg, 8.00 mmol), colorless solid).
- Dissolve 600 mg (0.908 mmol) of 14 (product obtained from step 2.1) in 15 ml dry tetrahydrofurane.
- Zemplén Deacetylation: Synthesis of (5-bromo-4-chloro-indol-3-yl)-β-ᴅ-galactopyranoside (16)
- Dissolve 396 mg (0.436 mmol) 15 (product obtained from step 2.2) in 10 ml methanol.
- Add a catalytic amount of sodium methanolate and stir overnight at room temperature.
Caution! Sodium methanolate is flammable and corrosive. - Neutralize with Amberlite IR-120 H+ (filter) and concentrate.
Caution! Amberlite IR-120 H+ is irritant. - Dry the product in vacuum, (yield: 87% (170 mg, 0.416 mmol), colorless solid).
3. Illustrating Model Experiment: Activity proof of β-galactosidase employing X-Gal
- Prepare a 0.5 mM solution of X-Gal in 500 μl buffer (Tris-HCl 20 mM; pH 7.4; 50 mM NaCl, 0.1 mM ethylenediaminetetraacetic acid; or any other suitable buffer for your enzyme) in an microcentrifuge tube (X-Gal can be dissolved in a few μl of dimethylsulfoxide).
- Add 1 μl β,1-3-galactosidase (EC 3.2.1.23 10,000 U/ml; or any other suitable β-galactosidase) and shake (600 rps) at 37 °C.
- Follow the reaction. The indigo type dye is formed after a short period of time.
Результаты
The very first syntheses of indicane and 5-bromo-indicane, were published by Robertson already in 1927 and 192915,16. By employing indoxylic acid methyl ester as acceptor, the reactive 2-position was blocked and thus side reactions were partially suppressed. Glycosylation in acetone/sodium hydroxide, following deprotection and decarboxylation (160 °C, acetic anhydride, 1 hr) and finally deacetylation yielded Indicane and 5-bromo-indicane. Based on this concept we developed an improved synthesis of indoxyl...
Обсуждение
Owing to poor yields and limitations, especially for glucose type structures and more complex saccharides, a novel synthetic approach towards indoxyl glycosides was developed. Indoxylic acid esters proved to be precious key intermediates and were obtained in a modular, scalable pathway. All steps are high yielding and due to cheap starting materials and easy workup multi-gram syntheses are possible. The advantage of the allyl ester approach is the blocking of the reactive 2-position. Thus yield decreasing side reactions ...
Раскрытие информации
The authors declare that they have no competing financial interests.
Благодарности
Support of this work by Glycom A/S, Copenhagen, Denmark, is gratefully acknowledged.
Материалы
| Name | Company | Catalog Number | Comments |
| Acetic anhydride | Grüssing | 10298 | Corrosive, flammable |
| Acetonitrile | Sigma-Aldrich | 608-001-00-3 | Harmful, flammable |
| Allyl alcohol | Aldrich | 453021 | Harmful, dangerous for the environment |
| Amberlite IR-120 H+ | Fluka | 06428 | Irritant |
| Bromoacetic acid | Merck | 802260 | Corrosive, toxic, dangerous for the environment |
| 4-Bromo-3-chloro-2-methylaniline | ABCR | AB 171687 | Irritant |
| Dichloromethane | ACROS | 326850010 | Harmful |
| Diethyl ether | Grüssing | 10274 | Harmful, extremly flammable |
| Dimethylformamide | ACROS | 348430010 | Harmful, flammable |
| Dimethylsulfoxide | Sigma-Aldrich | 41648 | |
| Ethyl acetate | Sigma-Aldrich | 607-022-00-5 | Irritant, flammable |
| Ethylenediaminetetraacetic acid | AppliChem | A1103.0500 | Irritant |
| β1,3-Galactosidase, recombinant, E. coli | Calbiochem | 345795 | |
| Hydrochloric acid | VWR | 20252.290 | Corrosive |
| Magnesium sulfate hydrate | Merck | 105885 | |
| Methanol | ACROS | 326950010 | Toxic, flammable |
| Morpholine | Janssen Chimica | 15.868.57 | Corrosive, flammable |
| Peroleum ether | Azelis | 111053 | Flammable, irritant, dangerous for the environment |
| Potassium carbonate | Grüssing | 12005 | Corrosive |
| Potassium permanganate | Grüssing | 12056 | Harmful, oxidising |
| Potassium tert-butoxide | Merck | 804918 | Corrosive, flammable |
| Pyridine | Sigma-Aldrich | 613-002-00-7 | Harmful, flammable |
| Silver acetate | Fluka | 85140 | Irritant, dangerous for the environment |
| Sodium bicarbonate | Grüssing | 12144 | Corrosive |
| Sodium hydride | Merck | 814552 | Corrosive, flammable |
| Sodium hydroxide | Riedel-de Häen | S181200 | Corrosive |
| Sodium methanolate | Merck | 806538 | Corrosive, flammable |
| Sodium sulfate | Grüssing | 12175 | |
| Tetrabutylammonium hydrogensulfate | Lancaster | 5438 | Harmful |
| Tetrahydrofurane | Sigma-Aldrich | 87371 | Harmful, flammable |
| Tetrakis (triphenylphosphine)palladium(0) | Sigma-Aldrich | 216666 | |
| Triphosgene | Fluka | 15217 | Toxic |
| Tris (hydroxymethyl)aminomethane hydrochloride | Sigma | T-3253 | Irritant |
Ссылки
- Clark, R. J. H., Cooksey, C. J., Daniels, M. A. M., Withnall, R. Indigo, Woad, and Tyrian Purple: Important Vat Dyes from Antique to the Present. Endeavour. 17, 191-199 (1993).
- Hunger, K. . Industrial Dyes: Chemistry, Properties, Applications. , (2003).
- Kiernan, J. A. Indigogenic Substrates for Detection and Localization of Enzymes. Biotechn. Histochem. 82, 73-103 (2007).
- Barnett, R. J., Seligman, A. M. Histochemical Demonstration of Esterases by Production of Indigo. Science. 114, 579-582 (1951).
- Pearson, B., Andrews, M., Grose, F. Histochemical Demonstration of Mammalian Glucosidase by Means of 3-(5-Bromoindolyl)-β-ᴅ-glucopyranoside. Exp. Biol. Med. 108, 619-623 (1961).
- Anderson, F. B., Leaback, D. H. Substrates for the Histochemical Localization of some Glycosidases. Tetrahedron. 12, 236-239 (1961).
- Van Dort, M. E., Lee, K. C., Hamilton, C. A., Rehemtulla, A., Ross, B. R. Radiosynthesis and Evaluation of 5-[125I]Iodoindolyl-3-yl-β-ᴅ-galactopyranoside ([125I]IBDG) as a β-Galactosidase Imaging Radioligand. Mol. Imaging. 7, 187-197 (2008).
- Yoshida, K., Iino, N., Koga, I. Syntheses of Halogen Substituted β-ᴅ-Glucuronides and Their Hydrolysis by Rabbit Liver β-Glucoronidase. Chem. Pharm. Bull. 32, 1759-1769 (1975).
- Horwitz, J. P., et al. Substrates for Cytochemical Demonstartion of Enzyme Activity I. Some Substituted 3-Indolyl-β-ᴅ-glycopyranosides. J. Med. Chem. 7, 574-575 (1964).
- Eschenfelder, V., Brossmer, R. 5-Bromo-indol-3-yl 5-Acetamido-3,5-dideoxy-α-ᴅ-glycero-ᴅ-galactononulopyranosidic Acid, a Novel Chromogenic Substrate for the Staining of Sialidase Activity. Glycoconjugate J. 4, 171-178 (1987).
- Fujii, I., Iwabuchi, Y., Teshima, T., Shiba, T., Kikuchi, M. X-Neu5Ac: A Novel Substrate for Chromogenic Assay of Neuraminidase Activity in Bacterial Expression Systems. Bioorg. Med. Chem. 1, 147-149 (1993).
- Berlin, W., Sauer, B. In situ Color Detection of α-ʟ-Arabinofuranisodase, a "No-Background" Reporter Gene, with 5-Bromo-3-indolyl-α-ʟ-arabinofuranoside. Anal. Biochem. 243, 171-175 (1996).
- Marmuse, L., et al. New Chromogenic Substrates for Feruloyl Esterases. Org. Biomol. Chem. 6, 1208-1214 (2008).
- Kaneko, S., Kiaoka, M., Kuno, A., Hayashi, K. Syntheses of 4-Methylumbelliferyl-β-ᴅ-Xylobioside and 5-Bromo-3-Indolyl-β-ᴅ-Xylobioside for Sensitive Detection of Xylanase Activity on Agar Plates. Biosci. Biotechnol. Biochem. 64, 741-745 (2000).
- Robertson, A. J. Syntheses of Glucosides. Part I. The Synthesis of Indican. Chem. Soc. , 1937-1943 (1927).
- Robertson, A., Waters, R. B. J. Synthese of Glucosides. Part VII. The Synthesis of 6-Bromoindican. Chem. Soc. , 2239-2243 (1929).
- Böttcher, S., Thiem, J. Indoxylic Acid Esters as Convenient Intermediates Towards Indoxyl Glycosides. Eur. J. Org. Chem. , 564-574 (2014).
- Böttcher, S., Hederos, M., Champion, E., Dékány, G., Thiem, J. Novel Efficient Routes to Indoxyl Glycosides for Monitoring Glycosidase Activities. Org. Lett. 15, 3766-3769 (2013).
- Böttcher, S., Thiem, J. Facile Preparation of Indoxyl- and Nitrophenyl Glycosides of Lactosamine and Isolactosamine. RSC Adv. 4, 10856-10861 (2014).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеThis article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены