Для просмотра этого контента требуется подписка на Jove Войдите в систему или начните бесплатную пробную версию.
Method Article
Echocardiographic Assessment Using Subxiphoid-Only Examination for Hypotensive Patients
В этой статье
Резюме
The protocol here provides a rapid, phenotype-based approach for focused ultrasound evaluation of the hypotensive patient, using subcostal images of the inferior vena cava and heart, with additional views of the upper lungs and pleural spaces.
Аннотация
Point-of-care ultrasound, or POCUS, has gathered significant interest as a tool to augment clinical decision-making in the care of acutely ill patients. Patients with hemodynamic instability, in particular, require immediate intervention and are at high risk for further cardiovascular and/or respiratory decompensation. POCUS examination of these patients, therefore, requires a protocol that is sufficient to answer clinicians' questions while still retaining adequate brevity to be feasible in a short period of time at the bedside. Here, we demonstrate a protocol for obtaining such information by the Echocardiographic Assessment using Subxiphoid-Only - Mean Arterial Pressure, or EASy MAP exam, a focused ultrasonographic assessment of the cardiovascular and respiratory systems. The cardiovascular portion of the examination utilizes the subcostal window to obtain views of the heart and inferior vena cava (IVC); this is then supplemented by anterior upper lung and posterolateral diaphragmatic pleural views for the respiratory portion. All six views (cardiac, IVC, left and right anterior upper lung, and left and right posterolateral pleural) are obtained using a low-frequency phased array transducer. We discuss some of the common pitfalls encountered in obtaining these images and the basics of interpreting the most common findings; also described is a 12-point scale for rating the quality of the images obtained from the cardiac view and a small number of supplementary views that may be considered when clinically relevant.
Введение
In recent years, the increased availability, quality, and portability of ultrasonographic equipment has driven a rapid expansion in the field of point-of-care ultrasound (POCUS). POCUS is defined as an ultrasound exam that is performed for well-defined clinical indications and is interpreted by the patient's primary treating clinician, with the findings immediately incorporated into the treatment plan1,2. This is in contrast to consultative ultrasound, which refers to an ultrasound exam that is requested by a patient's primary treating clinician but performed by a separate specialist team3. Because consultative exams must be transmitted to a reading expert after the acquisition of the images, there may be a significant lag time between the identification of the clinical indication and the availability of the study for clinical decision-making. Given that consultative exams require significant time to acquire all images and require the availability of both a dedicated sonographer and then a reading physician4, such exams cannot always be performed and read on the time scales demanded by acutely unstable patients (often minutes) and are not feasible in all clinical settings, especially during off hours (i.e., evenings, nights, weekends, and holidays). In contrast, portable ultrasound machines can be expediently brought to the bedside by treating clinicians, allowing POCUS exams to provide actionable information for immediate clinical decision-making. For example, POCUS can provide an immediate window into the hemodynamic status of a patient5,6,7, augmenting more traditional clinical tools such as physical examination, clinical gestalt, and other hemodynamic monitoring techniques. While cardiac POCUS uses the same basic ultrasound technology as more comprehensive evaluations by consultative cardiology echocardiographic studies, POCUS exams have their own specific clinical indications and can be obtained in a much wider range of emergency situations (trauma bay, operating room, acute stabilization in the post-anesthesia care unit, etc.) and in a short amount of time8 for immediate clinical decision-making. Fundamentally, POCUS studies are obtained for the purpose of answering a specific clinical question, the answer to which will guide the patient's immediate management.
Significant research has previously been directed towards the use of POCUS for evaluation of volume status; the size and collapsibility of the inferior vena cava (IVC) has often been studied as a proxy for determining the preload of the right heart, which, for some patient populations, is a useful estimator of volume status9,10,11. However, the utility of IVC evaluation alone as a predictor of response to fluid resuscitation is of less certain value in patients with undifferentiated shock, with substantial heterogeneity between studies and patient populations12,13,14,15; many critically ill patients are also receiving positive pressure ventilation, which can confound attempts to estimate central venous pressure via IVC ultrasound alone16. Additionally, given that the venous return comprises just one of many factors necessary for adequate cardiac output and end-organ perfusion, a systematic approach to the patient with acute clinical instability (such as hypotension or respiratory failure) is likely to require a more thorough picture of cardiovascular and respiratory status, necessitating the introduction of additional sonographic views. Patients with sepsis, for example, may exhibit a wide range of cardiac phenotypes17, meaning that an IVC-only approach would miss significant hemodynamic heterogeneity.
Here, we describe a method for rapidly evaluating the acutely hypotensive patient by the acquisition of an abbreviated POCUS exam: the Echocardiographic Assessment using Subxiphoid-only - Mean Arterial Pressure, or EASy MAP exam18. The EASy MAP exam is a concise examination for immediate determination of cardiovascular and cardiorespiratory status, consisting of subcostal views of the heart and IVC plus anterior and posterolateral lung ultrasound views. Within the EASy MAP protocol, IVC assessment is not intended to function in isolation but rather as one element of a broader framework that includes evaluation of cardiac function, lung ultrasound, and clinical context. The resultant images can then be interpreted using simple pattern recognition, with patients generally falling into one of several cardiovascular and pulmonary phenotypes; this information can then be used for the immediate management of the hypotensive patient. This integrated approach seeks to leverage pattern recognition to identify actionable clinical findings while acknowledging the inherent limitations of any single ultrasonographic metric. By simplifying the steps needed to obtain images and extract clinically actionable results, the goal is to broaden the range of clinicians who are able to use POCUS at the bedside and to simplify the knowledge transfer process and time to achieve competency18. These exams are performed with the understanding that quantitative measurements could still be required, triggering follow-up by consultative echocardiographic studies, especially if signs of chronic disease are identified during the EASy MAP exam. There are many circumstances where both EASy MAP evaluation and consultative examinations will be appropriate, with EASy MAP being performed as the most immediate evaluation and closed-loop reassessment of response to therapy.
For purposes of this examination and protocol, we define hypotension as a mean arterial pressure (MAP) less than 65 mmHg19,20,21,22,23, excluding patients in cardiac arrest. We describe a protocol for image acquisition (in accordance with previously established ultrasonographic techniques24,25). We also describe common pitfalls in image acquisition and methods for identification of the relevant anatomy. We also provide a standardized 12-point scoring system for the evaluation of the quality of cardiac images. The goal of this protocol is to allow clinicians to obtain immediate information about the etiology of the patient's hypotension such that the treatment plan can be adjusted to best address the patient's condition.
The viability of the EASy MAP protocol as an entry-level POCUS examination has been previously validated in a number of patient populations in a small cohort study18,26, novice sonographers with a single day of didactic training in a similar EASy protocol were able to acquire a clinically actionable image in 55 out of 63 total exams (87%; Figure 1). In 100 hypotensive patients with sepsis, EASy exam images were of sufficient quality to guide patient management in 75% of cases8, and phenotypes recognized on the EASy exam were associated with significant differences in patient management.
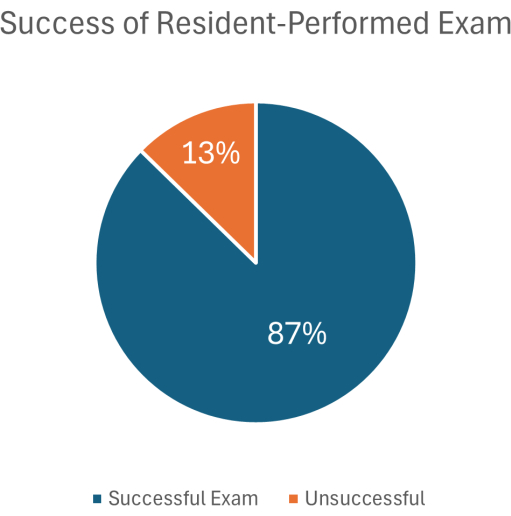
Figure 1: Success of EASy exams performed by trainees following a 1-day didactic. A total of 63 exams were performed on 14 unique patients over the course of 12 days. This figure has been modified from18. Please click here to view a larger version of this figure.
протокол
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Albany Medical Center institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All videos demonstrating the process of performing the EASy MAP examinations and all examples of normal (i.e., non-pathologic) ultrasound images depict the authors themselves or, in some cases, healthy volunteers who gave written consent for involvement in the filming process. The protocol described in this publication has been developed for the evaluation of adult patients with arterial hypotension in an acute care setting; its use in pediatric populations has not been closely studied.
1. Patient selection
- Inclusion criteria
- Examine the patient's vital signs. If the mean arterial pressure (MAP) is less than 65 mmHg, ensure that no errors in measurement have occurred.
- If the patient is confirmed to be hypotensive (MAP < 65 mmHg), perform EASy POCUS exam for assessment of the patient's hypotension.
NOTE: This publication and protocol do not cover patients in cardiac arrest who are actively undergoing chest compressions. Additionally, there may be specific situations in which the clinician may elect to use a different blood pressure cutoff value, but these are outside the scope of this protocol.
- Exclusion criteria
- If the patient is unable to lay flat or is unable to tolerate pressure on the subcostal region, consider alternative means of hemodynamic assessment.
- If the patient has a body habitus that is likely to result in significant degradation of ultrasound image quality (i.e., extreme obesity), consider alternative means of hemodynamic assessment.
2. Clinical safety
- Observe standard precautions according to institutional and local protocol for infection control and don any relevant PPE.
- If the patient is at elevated risk of invasive infections of the thoracic or abdominal cavity, consider applying a sterile probe cover and using sterile ultrasound gel on a case-by-case basis.
3. Probe selection
- Select a linear sector array (commonly referred to as cardiac or phased array) probe for all portions of the examinations.
NOTE: The exam can also be conducted using a curvilinear probe, which offers greater penetration at the cost of a larger footprint and significantly lower temporal (motion) resolution. The sector array probe provides adequate tissue penetration, and the smaller profile of the probe allows for imaging of the thoracic organs without excessive rib shadowing. Given that many other ultrasound probes make use of phasing for beam control27, we prefer to refer to avoid referring to the cardiac or sector array probe as a phased array probe in formal contexts. - Ensure the probe is clean and free of contaminants. If it is contaminated with gel residue, clean it with an antimicrobial cleaning wipe before performing the examination.
4. Machine presets and image orientation
NOTE: Many directions in this manuscript regarding buttons and onscreen commands are relatively specific to each model of the bedside ultrasound machine. We have used language for one specific model here; the exact sequence of on-screen buttons, commands, preset names, etc., will vary with manufacture and model.
- Touch the Power button to turn on the machine. Touch the Probe button on the bottom left of the screen and select Adult Cardiac for the probe preset. Ensure that B is selected on the right of the screen for B-mode imaging; if it is not highlighted, touch it to enter B-mode.
- After entering the Adult Cardiac preset, ensure that the marker indicator adheres to the cardiology convention - that is, it appears on the upper right side of the screen.
- Use the slider on the right of the screen to adjust the depth to 21 cm.
- Touch the Image Quality button on the left of the screen, if present, and ensure that the frequency is set to the lowest frequency of the probe (typically 2 MHz).
- Touch the TGC button and ensure that time gain compensation is switched on; increase the far gain to improve the image in the far field.
NOTE: The core images are acquired primarily as B-mode 2D grayscale images, and it is these images described in the protocol here. In some cases, it may be appropriate to perform additional ad hoc exam steps outside of the base protocol using tools such as color Doppler or M-mode; these are outside the core protocol and should be performed at the examining clinician's discretion.
5. Image acquisition
- Apply ultrasound gel to the transducer. Position the patient in a supine position, if tolerated and practical.
NOTE: The entire exam can be performed even when the patient is in a non-optimal position (e.g., sitting, not lying flat, etc.). However, caution should be exercised when interpreting IVC findings in these cases. - Perform acquisition of the four chambers subcostal cardiac view as described below24.
- Place the probe 2 cm inferior to the xiphoid process (Figure 2, position A) with the marker facing towards the patient's left (3 o'clock); this is best performed using an overhand grip.
- Trap a small skin fold with the probe to help ensure good contact between probe and skin; significant inward pressure may be required for satisfactory image quality. For conscious or lightly sedated patients, attend to patient comfort as needed.
- To better align the probe with the heart's position, angle17 the probe tail caudally and toward the patient's right, moving slightly out of the axial plane to direct the ultrasound beam cephalad and toward the patient's left. This technique typically leverages the left lobe of the liver to enhance beam transmission, so a segment of the liver should appear in the superficial portion of the image.
- If necessary, increase the depth using the slider on the right of the screen to capture the entire heart. The view should extend slightly beyond the posterior wall of the LV (Figure 3 left image) to ensure that the entire pericardium is included.
- Adjust the gain using the gain slider such that the blood filling the chambers appears black. Some visible turbulent flow is acceptable.
NOTE: In certain cases, such as extremely low flow states or mechanical circulatory support with minimal native output, intraventricular coagulation and/or cellular clumping may occur, resulting in a swirling, heterogeneous echogenicity within the ventricles. - Assess the quality of the image. The correctly acquired image will visualize both ventricles, both atria, and the tricuspid and mitral valves, but not the left ventricular outflow tract.
- Once the heart has been adequately visualized, press Save Clip on the ultrasound screen to acquire a 4 s video clip.
NOTE: The 4 s duration is the typical default clip length for the equipment used for the videos that accompany this publication, and this length generally allows the capture of multiple cardiac cycles at most heart rates. If equipment allows, the examiner may consider using a longer clip length in certain cases, for example, if the ventricular rate is < 30 bpm.
- Perform acquisition of the subcostal IVC view as described below24.
- Remove the probe entirely from the subcostal area (Figure 2, Position B) and rotate it so that the marker points cephalad (toward 12 o'clock). Reposition the probe on the subcostal area at the same location used for the cardiac views but with a new orientation to visualize the IVC (Figure 3 right image). In this view, the IVC will be within the liver and can typically be seen draining into the right atrium of the heart in a cephalad direction.
NOTE: Removing the probe from the patient's chest entirely helps prevent mistaking the right hepatic vein for the IVC. Both underhand and overhand grips are suitable for this view. - Angle the probe to direct the beam slightly toward the patient's left (probe tail angled toward the patient's right) to locate the aorta. The aorta will be immediately to the patient's left of the IVC, and vice versa. Since the abdominal aorta and IVC are adjacent and have similar ultrasonographic appearances, it is crucial to positively identify both structures to avoid mistaking the aorta for a non-collapsing IVC.
NOTE: Identification of the hepatic vein draining into the IVC from the hepatic parenchyma can also be used as a method of IVC identification; however, we prefer positive identification of the aorta, given that this can be performed reliably in essentially all patients. - Once the IVC has been identified and adequately visualized, make a note of the maximum size (i.e., diameter) and inspiratory collapsibility (or distensibility for the patient on positive pressure ventilation). Manual measurements are the preferred standard, but the ultrasound machine's automated IVC analysis tool may also be used.
- Press Save Clip on the ultrasound screen to acquire a 4 s video clip of the IVC.
- Press Save Clip on the ultrasound screen to acquire a 4 s video clip of the aorta.
NOTE: Beyond the core protocol, additional optional views can be obtained after identifying the aorta. Rocking the probe tail inferiorly allows visualization of the aortic valve while rocking it inferiorly and tilting it toward the patient's right, with rotation towards the patient's left shoulder, revealing the midpapillary short-axis view. If assessing gastric volume or fasting status is necessary, the gastric antrum can be visualized by rocking the probe tail cephalad from the aorta.
- Remove the probe entirely from the subcostal area (Figure 2, Position B) and rotate it so that the marker points cephalad (toward 12 o'clock). Reposition the probe on the subcostal area at the same location used for the cardiac views but with a new orientation to visualize the IVC (Figure 3 right image). In this view, the IVC will be within the liver and can typically be seen draining into the right atrium of the heart in a cephalad direction.
- Acquisition of the upper lung ultrasound views
- Remove the probe from the subcostal position; reapply the ultrasound gel if needed. Wipe any excess gel off the patient's abdomen.
- Place the probe in the 2nd intercostal space at the right midclavicular line, with the probe marker in a 12 o'clock position (Figure 4 left image).
- Apply gentle pressure to the probe and/or vertically slide the probe as necessary to minimize the degree to which the view is obscured by rib shadowing, which appears as vertical acoustic shadows created by the dense cortex of the ribs.
- The pleura, acting as a specular reflector of ultrasound, is best visualized when the ultrasound beam is perpendicular to its surface. To optimize this, tilt the probe tail slightly laterally to account for the natural curvature of the chest wall.
- Adjust the gain such that the airspaces of the lung parenchyma appear black (Figure 5 right).
- If image quality is poor, touch the Probe button on the bottom left of the screen and select Adult Lung for the probe preset. Repeat 5.4.5 as needed.
NOTE: Changing from a cardiac to a lung ultrasound preset moves the probe marker indicator to the left side of the screen and reverses the orientation of the image. - Once a satisfactory view has been obtained between the ribs, press the Save Clip on the screen to obtain a video clip for later analysis.
- Repeat the process (5.4.2-5.4.4) with the left upper lung, placing the probe in the 2nd intercostal space at the left midclavicular line (Figure 2 position D).
- Acquisition of the pleural ultrasound views
- Wipe any excess gel off the anterior chest. Reapply gel to the probe as needed.
- Place the probe at the right midaxillary line (Figure 5 right image) in the same horizontal plane as the subxiphoid cardiac view. Orient the probe with the marker in the 12 o'clock position (i.e., directed superiorly).
- Identify the liver (Figure 5 left image), then angle the probe to direct the beam in a more cephalad direction and identify the diaphragm, which should be seen as a curved hyperechoic band immediately cephalad to the liver. In some cases, sliding the probe more posteriorly may be necessary to improve the beam transmission window through the hepatic parenchyma.
- Angle the tail of the probe in an anterior direction to Identify the anterior surface of the spine, visible at the bottom of the screen. Since the ultrasound beam cannot penetrate bone effectively, the spine will appear as a hyperechoic line with dark shadowing beyond it.
- Identify the lung, which should sweep a large acoustic shadow across the screen (from left to right) with inspiration.
- Once the liver, diaphragm, spine, and lung are all brought into view simultaneously, press Save Clip on the ultrasound screen to capture a video clip for later analysis.
- Reposition the probe to the left posterior axillary line, in the same horizontal plane as the subcostal cardiac view, with the marker in the 12 o'clock position (Figure 6 right image).
- Identify the spleen (Figure 6 left image). If there is difficulty visualizing the spleen due to gastric air or patient anatomy, it may be helpful to slide the probe posteriorly (to the extent that the examination table and patient's chest wall permit) and identify the left kidney, which generally lies inferior to the spleen.
- Identify the left hemidiaphragm superior to the spleen. As on the right, it should appear as a bright hyperechoic line.
- Identify the spine and lung, as in 5.5.3 and 5.5.5. Once the spleen, diaphragm, spine, and lung are all brought into view simultaneously, press the Video button, then hit Save Clip on the ultrasound touchscreen to capture a video clip for later analysis.
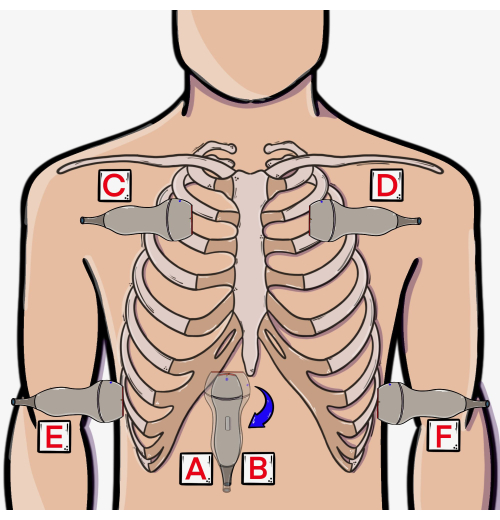
Figure 2: Approximate probe positions for EASy protocol steps. (A) Subcostal cardiac, (B) subcostal IVC, (C, D) lung, and (E, F) pleural views. Abbreviations: RUL = right upper lobe, LUL = left upper lobe, RML = right middle lobe, RLL = right lower lobe, LLL = left lower lobe. This figure has been modified from36. Please click here to view a larger version of this figure.

Figure 3: Normal heart in subcostal 4-chamber cardiac view (left) and normal IVC as viewed in subcostal IVC view (right). Please click here to view a larger version of this figure.
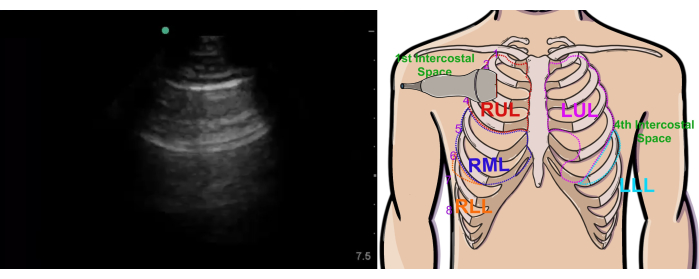
Figure 4: Normal right upper lung ultrasound view and probe position. The figure shows the ultrasound view (left) and probe position (right). Abbreviations: RUL = right upper lobe, LUL = left upper lobe, RML = right middle lobe, RLL = right lower lobe, LLL = left lower lobe. This figure has been modified from36. Please click here to view a larger version of this figure.
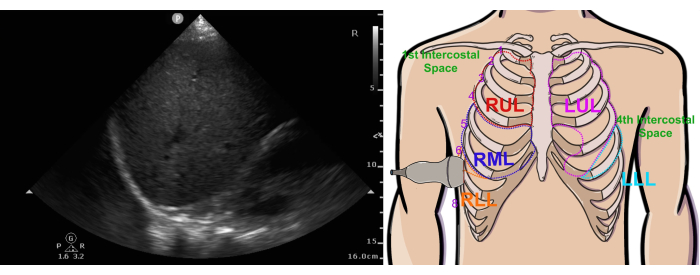
Figure 5: Normal right pleural ultrasound view and probe position. The figure shows the ultrasound view (left) and probe position (right). Abbreviations: RUL = right upper lobe, LUL = left upper lobe, RML = right middle lobe, RLL = right lower lobe, LLL = left lower lobe. This figure has been modified from36. Please click here to view a larger version of this figure.
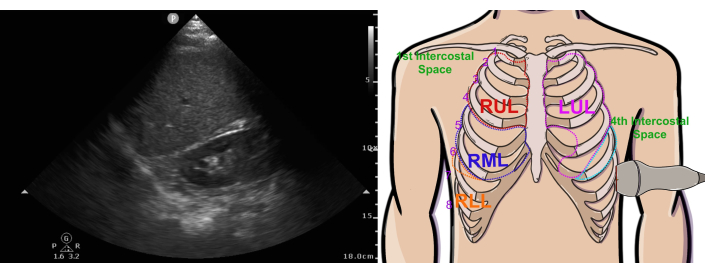
Figure 6: Normal left pleural ultrasound view. Abbreviations: RUL = right upper lobe, LUL = left upper lobe, RML = right middle lobe, RLL = right lower lobe. This figure has been modified from36. Please click here to view a larger version of this figure.
6. Image interpretation
- Interpretation of IVC images
- Evaluate the IVC on two major criteria - diameter and inspiratory collapsibility.
NOTE: For purposes of this protocol, a normal IVC diameter is defined as 0.9-2.1 cm in diameter, as measured during expiration; a distended IVC is defined as > 2.1 cm and a flat IVC < 0.9 cm24,28,29. A patient's IVC is described as collapsible if it exhibits > 50% collapse during inspiration; an IVC that has < 50% collapse is described as plethoric. These criteria require that the patient be breathing spontaneously, without the use of positive pressure ventilation. These numeric cutoffs are intended for a patient not receiving positive pressure ventilation (PPV). PPV can significantly confound the evaluation of the IVC due to increased intrathoracic pressure, which reduces size variability. A diameter variability cutoff of 15% (rather than 50%) may be considered for patients on PPV when considering the right heart preload and volume responsiveness of the patient16,30,31,32. - Based on these two characteristics, sort patients into one of three categories: poor venous return (flat, collapsible IVC), poor forward flow (distended, plethoric IVC), or none of the above (IVC does not meet either of the two preceding criteria and thus is grossly normal).
NOTE: Overall diagnostic interpretation and management will depend on the patient's clinical scenario and the information obtained from the other views.
- Evaluate the IVC on two major criteria - diameter and inspiratory collapsibility.
- Interpretation of subcostal cardiac images
- Record the overall quality of the cardiac images by assigning each of the six cardinal elements (pericardium, RV size, RV function, interventricular septum, LV size, LV function) a score of 0, 1, or 2.
- Award a score of 0 if the respective characteristics cannot be assessed from the images obtained or if the structure was not visualized at all; award a score of 1 for suboptimal but usable images, which require additional cardiac views to obtain a satisfactory clinical picture; and a score of 2 for high-quality images that provide a clear assessment of the respective structures.
- Sum the scores of all 6 cardinal elements of the heart (pericardium, RV size, RV function, interventricular septum, LV size, LV function) to obtain the total score. Consider a total score of 10-12 a high-quality cardiac view, 7-9 adequate, and 6 or fewer a poor-quality image.
NOTE: Images which are rated as poor are unlikely to be usable for clinical decision making, although the other elements of the exam (i.e. IVC, lungs, pleura) may still be actionable. - Evaluate the heart in a systematic fashion, utilizing a standardized ordering of structures and identifying specific patterns (phenotypes).
- Pericardium: Assess for the presence of non-trivial pericardial fluid, defined in this protocol as an effusion >10 mm that is visible throughout systole and diastole. The pattern of cardiac tamponade (phenotype 8) is a clinical diagnosis, supported by sonographic signs of elevated intrapericardial pressure, such as atrial collapse during systole or ventricular collapse during diastole33.
- Right ventricle: Assess the size and function of the right ventricle (RV). Minimal reduction in RV cavity size during systole and depressed tricuspid annular motion indicate impaired RV systolic function. If the RV is severely dilated (RV/LV size ratio > 1) evaluate for signs of underlying chronic dysfunction, such as RV wall thickness during diastole exceeding 1 cm or visible enlargement of the right atrium. (Phenotypes 6 and 7, Figure 7).
- Interventricular Septum: Evaluate the thickness of the interventricular septum during diastole, checking for thinning, rupture, or hypertrophy. If RV dilation is observed, assess for interventricular septal shift toward the LV.
- Left ventricle: Evaluate the size and function of the left ventricle (LV), identifying any severe dilation (Phenotypes 4 and 5, Figure 7) or significant wall thickening (Phenotype 3). Assess for severely reduced systolic function, focusing on visually apparent abnormalities at this stage of the protocol. Severe systolic dysfunction is indicated by minimal or no reduction in LV cavity size during systole, marked reduction of the mitral valve annulus, and anterior mitral valve leaflet movement.
NOTE: Severely increased wall thickness (concentric hypertrophy) may confound the assessment of hypovolemia due to decreased cavity size. The examiner may optionally perform quantitative assessments of systolic and diastolic function, but these are outside the scope of this particular protocol.
- After reviewing the cardiac images, integrate this data with information about preload conditions (from the IVC exam), volume tolerance (e.g., presence or absence of B-lines on upper lung ultrasound), and the patient's clinical context. This combined assessment helps determine the overall cardiovascular status and guides subsequent management. Based on these findings, categorize patients into specific cardiovascular phenotypes (Figure 7).
- To assess for a distributive hemodynamic pattern (Phenotype 2), check for adequate end-diastolic area of the LV and RV, hypercontractile or hyperdynamic systolic function in both the RV and LV (>50% reduction in ventricular cavity size during systole), normal systolic thickening (>30%), and robust movement of the mitral valve annulus and anterior mitral valve leaflet. Additional features include a normal IVC diameter with appropriate respiratory variability and an A-profile on lung ultrasound.
- Check the following indicators for a hypovolemic hemodynamic pattern (HD Phenotype 1): the end-diastolic areas of the LV and RV are small, often accompanied by a flat IVC.
- If the subcostal cardiac images indicate chronic cardiovascular disease (e.g., increased wall thickness, atrial enlargement, significant valvular disease, or hypokinesis), obtain a formal consultative echocardiogram as soon as possible.
- Record the overall quality of the cardiac images by assigning each of the six cardinal elements (pericardium, RV size, RV function, interventricular septum, LV size, LV function) a score of 0, 1, or 2.
- Interpretation of upper lung images25
- Determine the presence of A lines and/or B lines in the right and left lungs. If neither A lines nor B lines are seen, recheck the probe orientation and image quality. A lack of either A or B lines suggests that the probe is not oriented at the correct angle relative to the pleurae.
- Assess for the presence of lung sliding. Lung sliding is seen as the lateral movement of the two pleural layers relative to one another during the respiratory cycle34.
- Interpretation of Pleural images
- Assess the presence of pleural effusion (or other excess pleural fluid, such as hemothorax in trauma patients) in the right and left lungs. If effusion is present, make note of any loculations.
- Assess for the presence of any consolidations, which may be seen either directly (i.e., as hyperechoic patchiness within the lung parenchyma) or more indirectly (i.e., via a spine sign or loss of the airspace curtain)35.
7. Common etiologies of hypotension and EASy exam findings
- Monitoring and clinical context
- Consider the patient's vital signs and any electrocardiographic data, such as monitor tracings or formal EKGs, assessing the presence of any new onset of severe tachycardia, bradycardia, or dysrhythmia.
- If a new onset of severe bradycardia or dysrhythmia such as ventricular tachycardia, atrioventricular reentrant tachycardia, or atrial fibrillation with rapid ventricular response is noted, consider these first before considering other causes of hypotension.
- Consider clinical history, the nature of the present illness, and any recent surgery or other procedures when interpreting images and assessing the etiology of hypotension. While there are more edge cases than can be reasonably described in a single protocol, particular attention should be paid to any history of recent traumatic injury, abdominal, pelvic, or thoracic surgeries, or severe lung disease, as these can significantly affect venous return and pulmonary vascular resistance.
- Assessment of stroke volume
- If ultrasonographic assessment reveals a hemodynamic pattern of flat, collapsing IVC (poor venous return) and a hyperdynamic heart with small ventricular cavities (consistent with HD Phenotype 1 in Figure 7), consider this as low stroke volume due to hypovolemia.
- Note that in some cases, poor venous return will have etiologies other than intravascular volume depletion per se; in patients with increased intra-abdominal pressure-such as from a gravid uterus, abdominal compartment syndrome, or excessive insufflation during laparoscopic or robotic surgery-consider intra-abdominal obstructive physiology as a potential cause for low stroke volume and hypotension. Check for the presence of pulmonary A-lines, which will further support the diagnosis of hypovolemia.
- If ultrasonographic assessment revealed a dilated, plethoric IVC (poor forward flow) and a dilated, severely hypokinetic LV, make a presumptive diagnosis of cardiogenic shock (pump failure). Check for the presence of line B on pulmonary examination and/or simple pleural effusion, which will further support this diagnosis; see Cluster 2 phenotypes in Figure 7.
- If the ultrasonographic assessment revealed a plethoric IVC without obvious LV dilation, quickly evaluate and exclude potential intrathoracic obstructive causes of hypotension. These may include pericardial tamponade, acute or acute-on-chronic cor pulmonale, severe valvular disease, tension pneumothorax, auto-PEEP, or large pleural effusion or hemothorax (see Figure 7 and Figure 8).
NOTE: Many of the above diagnoses may be associated with supporting findings on the lung and pleural portions of the exam, such as the detection of large quantities of pleural fluid or the lack of lung sliding in tension pneumothorax. Obstructive causes of shock and hypotension can significantly impact the maximum diameter and variation of the IVC, irrespective of intravascular volume status.
- If ultrasonographic assessment reveals a hemodynamic pattern of flat, collapsing IVC (poor venous return) and a hyperdynamic heart with small ventricular cavities (consistent with HD Phenotype 1 in Figure 7), consider this as low stroke volume due to hypovolemia.
- Vasodilatory state/vascular tone
- If the EASy MAP exam does not reveal obviously abnormal ultrasonographic findings, yet the patient remains hypotensive, consider an arterial vasodilatory state as a potential diagnosis (Figure 7, Phenotype 2).
- If the only abnormal finding identified in the EASy MAP exam is a dilated IVC withoutpump failure, and intrathoracic causes of obstruction have been excluded, consider a diagnosis of vasodilation. The IVC may also be dilated in patients with a recent history of portosystemic shunt placement.
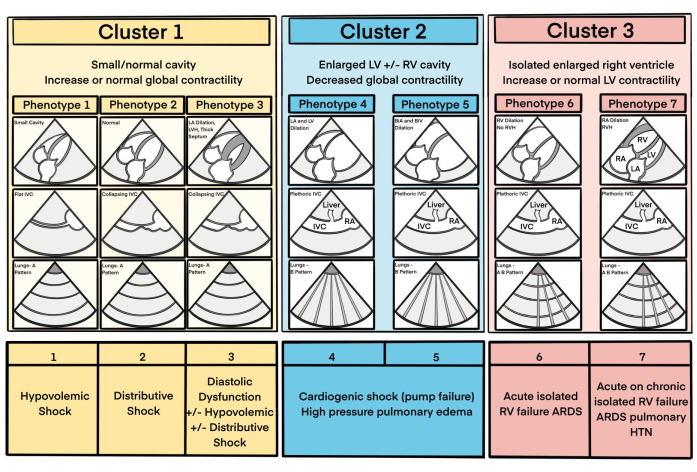
Figure 7: A schematic of several common cardiac IVC/phenotypes and associated disease processes/clinical scenarios. This figure has been modified from6. Please click here to view a larger version of this figure.
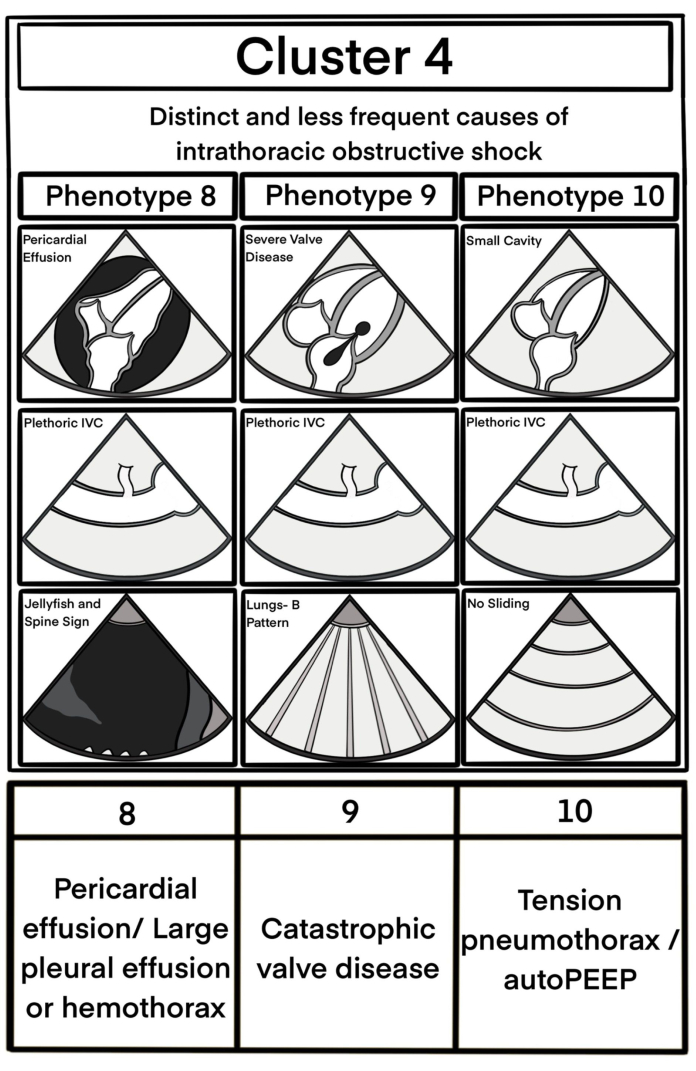
Figure 8: Uncommon phenotypes identified less frequently. This figure has been modified from6. Please click here to view a larger version of this figure.
Результаты
When the EASy protocol is used to assess the patient with hypotension, a successful exam is broadly defined as an exam that clarifies the diagnostic picture (in this case, the underlying etiology of hypotension) to a degree that is sufficient to immediately guide patient management. Conversely, an unsuccessful exam is one that is unhelpful in guiding management and does not provide sufficient information to result in actionable recommendations. High-quality imaging is essential for a successful EASy MAP examination, with...
Обсуждение
The EASy MAP protocol is just one of several ultrasound protocols that can be clinically useful in the evaluation of a patient with acute hemodynamic instability37,38. Unlike many more complex exams, however, the core of the EASy exam is its brevity and simplicity, which allows the user to rapidly identify many common pathologies at the bedside through pattern recognition18. The pattern recognition emphasis allows for simplified knowledge ...
Раскрытие информации
The authors have no relevant disclosures.
Благодарности
The authors wish to acknowledge the leadership of Albany Medical College for their support of students working under the auspices of the Summer Research Fellowship.
Материалы
| Name | Company | Catalog Number | Comments |
| Phased Array Ultraosund Probe | Mindray | P4-2S | Probe used for all components of exam |
| Portable Ultrasound System | Mindray | TE7 | Portable/bedside POCUS unit |
| Ultrasound Gel | Aquasonic | PLI 01-08 | Aqueous sonographic conduction gel |
Ссылки
- Bronshteyn, Y. S., Blitz, J., Hashmi, N., Krishnan, S. Logistics of perioperative diagnostic point-of-care ultrasound: Nomenclature, scope of practice, training, credentialing/privileging, and billing. Int Anesthesiol Clin. 60 (3), 1-7 (2022).
- Diaz-Gomez, J. L., Mayo, P. H., Koenig, S. J. Point-of-care ultrasonography. N Engl J Med. 385 (17), 1593-1602 (2021).
- Kirkpatrick, J. N., et al. Recommendations for cardiac point-of-care ultrasound nomenclature. J Am Soc Echocardiogr. 11 (24), (2024).
- Picard, M. H., et al. American society of echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 24 (1), 1-10 (2011).
- Bughrara, N., Diaz-Gomez, J. L., Pustavoitau, A. Perioperative management of patients with sepsis and septic shock, part ii: Ultrasound support for resuscitation. Anesthesiol Clin. 38 (1), 123-134 (2020).
- Nikravan, S., et al. An echocardiographic approach for the management of shock: The subcostal to apical, respiratory to parasternal-cardiac to respiratory, aortic to stomach protocol. Semin Ultrasound CT MR. 45 (1), 74-83 (2024).
- Nikravan, S., Song, P., Bughrara, N., Diaz-Gomez, J. L. Focused ultrasonography for septic shock resuscitation. Curr Opin Crit Care. 26 (3), 296-302 (2020).
- Bughrara, N., et al. 1479: Rapid ultrasound evaluation of patients with sepsis-associated hypotension using pattern recognition. Crit Care Med. 52 (S), S710 (2024).
- Caplan, M., et al. Measurement site of inferior vena cava diameter affects the accuracy with which fluid responsiveness can be predicted in spontaneously breathing patients: A post hoc analysis of two prospective cohorts. Ann Intensive Care. 10 (1), 168 (2020).
- Ginghina, C., Beladan, C. C., Iancu, M., Calin, A., Popescu, B. A. Respiratory maneuvers in echocardiography: A review of clinical applications. Cardiovasc Ultrasound. 7, 42 (2009).
- Griffin, M., et al. Inferior vena cava diameter measurement provides distinct and complementary information to right atrial pressure in acute decompensated heart failure. J Card Fail. 28 (7), 1217-1221 (2022).
- Long, E., Oakley, E., Duke, T., Babl, F. E. Paediatric Research in Emergency Departments International, C. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: A systematic review and meta-analysis. Shock. 47 (5), 550-559 (2017).
- Orso, D., et al. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: A systematic review and meta-analysis. J Intensive Care Med. 35 (4), 354-363 (2020).
- Preau, S., et al. Diagnostic accuracy of the inferior vena cava collapsibility to predict fluid responsiveness in spontaneously breathing patients with sepsis and acute circulatory failure. Crit Care Med. 45 (3), e290-e297 (2017).
- Yao, B., Liu, J. Y., Sun, Y. B., Zhao, Y. X., Li, L. D. The value of the inferior vena cava area distensibility index and its diameter ratio for predicting fluid responsiveness in mechanically ventilated patients. Shock. 52 (1), 37-42 (2019).
- Vignon, P., et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 195 (8), 1022-1032 (2017).
- Geri, G., et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 45 (5), 657-667 (2019).
- Bughrara, N. F., et al. Is 1 day of focused training in echocardiographic assessment using subxiphoid-only (easy) examination enough? A tertiary hospital response to the COVID-19 crisis and the use of the easy examination to support unit-wide image acquisition. Crit Care Explor. 6 (3), e1038 (2024).
- Evans, L., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47 (11), 1181-1247 (2021).
- Maheshwari, K., et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 44 (6), 857-867 (2018).
- Sarkar, S., Singh, S., Rout, A. Mean arterial pressure goal in critically ill patients: A meta-analysis of randomized controlled trials. J Clin Med Res. 14 (5), 196-201 (2022).
- Schenk, J., et al. Definition and incidence of hypotension in intensive care unit patients, an international survey of the European society of intensive care medicine. J Crit Care. 65, 142-148 (2021).
- Asfar, P., et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 370 (17), 1583-1593 (2014).
- Mitchell, C., et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American society of echocardiography. J Am Soc Echocardiogr. 32 (1), 1-64 (2019).
- Pereira, R. O. L., et al. Point-of-care lung ultrasound in adults: Image acquisition. J Vis Exp. (193), e64722 (2023).
- Bughrara, N., Quaranta, N., Pustavoitau, A., Shah, Q. Preintubation echocardiographic assessment using subcostal-only view (easy) exam: A case series. Crit Care Med. 50 (1), 696-696 (2022).
- Fischetti, A. J., Scott, R. C. Basic ultrasound beam formation and instrumentation. Clin Tech Small Anim Pract. 22 (3), 90-92 (2007).
- Di Nicolo, P., Tavazzi, G., Nannoni, L., Corradi, F. Inferior vena cava ultrasonography for volume status evaluation: An intriguing promise never fulfilled. J Clin Med. 12 (6), 2217 (2023).
- Lang, R. M., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 28 (1), 1-39.e14 (2015).
- Feissel, M., Michard, F., Faller, J. P., Teboul, J. L. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 30 (9), 1834-1837 (2004).
- Levitov, A., et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: Cardiac ultrasonography. Crit Care Med. 44 (6), 1206-1227 (2016).
- Schefold, J. C., et al. Inferior vena cava diameter correlates with invasive hemodynamic measures in mechanically ventilated intensive care unit patients with sepsis. J Emerg Med. 38 (5), 632-637 (2010).
- Klein, A. L., et al. American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: Endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography. J Am Soc Echocardiogr. 26 (9), 965-1012.e15 (2013).
- Bhoil, R., Ahluwalia, A., Chopra, R., Surya, M., Bhoil, S. Signs and lines in lung ultrasound. J Ultrason. 21 (86), e225-e233 (2021).
- Fox, W. C., Krishnamoorthy, V., Hashmi, N., Bronshteyn, Y. S. Pneumonia: Hiding in plain (film) sight. J Cardiothorac Vasc Anesth. 34 (11), 3154-3157 (2020).
- . . Master the Machines. , (2024).
- Nagre, A. S. Focus-assessed transthoracic echocardiography: Implications in perioperative and intensive care. Ann Card Anaesth. 22 (3), 302-308 (2019).
- Richards, J. R., Mcgahan, J. P. Focused assessment with sonography in trauma (fast) in 2017: What radiologists can learn. Radiology. 283 (1), 30-48 (2017).
- Sanfilippo, F., La Via, L., Flower, L., Madhivathanan, P., Astuto, M. The value of subcostal echocardiographic assessment, and directions for future research. Can J Anaesth. 69 (5), 676-677 (2022).
- Bughrara, N., et al. Comparison of qualitative information obtained with the echocardiographic assessment using subcostal-only view and focused transthoracic echocardiography examinations: A prospective observational study. Can J Anaesth. 69 (2), 196-204 (2022).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеСмотреть дополнительные статьи
This article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены