Method Article
Проект водно-болотных угодий в масштабе мезокосма для очистки сточных вод
* Эти авторы внесли равный вклад
В этой статье
Резюме
Построенные системы очистки водно-болотных угодий использовались в течение десятилетий для очистки сточных вод, но их применение для очистки вод, пострадавших от нефтеносных песков, является относительно новым. Для изучения этого потенциала была предложена разработка мезокосма поверхностного потока и экспериментальные методы. Этот подход направлен на улучшение нашего понимания ключевых параметров дизайна и повышение эффективности лечения.
Аннотация
Вода из нефтеносных песков, подверженная технологическому воздействию (OSPW), побочный продукт добычи битума открытым способом в провинции Альберта, Канада, содержит различные компоненты, вызывающие обеспокоенность, в том числе соединения фракции нафтеновой кислоты (NAFC). Эти органические соединения вызывают особое беспокойство из-за их токсичности и стойкости в окружающей среде. Сконструированные системы очистки водно-болотных угодий (CWTS) используют растения и связанные с ними микробы для снижения содержания загрязняющих веществ в сточных водах. Полевые CWTS были представлены в качестве потенциального широкомасштабного варианта лечения OSPW, в частности, для деградации NAFC. Для оптимизации использования CWTS для широкомасштабного лечения NAFC при OSPW важно углубить наше понимание различных параметров дизайна и изучить способы повышения эффективности.
Эксперименты мезокосмического масштаба служат ценным посредником, преодолевая разрыв между сложными полевыми испытаниями и контролируемыми лабораторными условиями. Мезокосмы обеспечивают контролируемую, воспроизводимую среду для изучения влияния различных параметров, таких как субстрат, виды растений, температура и время удержания, при этом учитывая экологические сложности при их проектировании. Опубликованные и предыдущие работы показали, что этот метод является успешным в оценке влияния различных параметров на эффективность CWTS для ослабления NAFC при OSPW. В этом протоколе описывается проектирование и создание мезокосма водно-болотных угодий с поверхностным стоком, а также экспериментальный подход к обработке NAFC в OSPW. Этот метод может быть адаптирован для очистки других сточных вод в различных географических точках.
Введение
Регион нефтеносных песков на севере провинции Альберта, Канада, содержит третьи по величине запасы нефти в мире, производя более 3 миллионов баррелей сырой нефти вдень1. Тем не менее, при добыче битума открытым способом образуются значительные объемы хвостов и воды, подверженной технологическому воздействию нефтеносных песков (OSPW) в качестве побочных продуктов. В связи с политикой нулевого сброса в Альберте, эти побочные продукты хранятся в хвостохранилищах по всему району добычи нефтеносных песков. По состоянию на 2023 год, по оценкам, 391,1мм3 OSPW существует в виде свободной воды в хвостохранилищах и не включает поровую воду, которая будет продолжать сбрасываться во время отсчета хвостохранилища2. OSPW содержит <5% твердых веществ и характеризуется повышенным уровнем солей, следов металлов, а также органических загрязнителей3.
В OSPW присутствует несколько основных классов загрязняющих веществ, включая соединения фракции нафтеновой кислоты (NAFCs), полициклические ароматические углеводороды (PAHs), BTEX (бензол, толуол, этилбензол и ксилолы), фенолы и тяжелые металлы 3,4. NAFC представляют собой органические соединения в битуме, которые растворяются и концентрируются в процессе экстракции и неизменно идентифицируются как основной источник острой токсичности OSPW 5,6. OSPW создают ряд экологических и экономических проблем из-за объема, сложности и токсичности смеси. Разработка экономически эффективных, пассивных и масштабируемых технологий очистки для OSPW имеет решающее значение, поскольку традиционные методы, такие как химическое окисление и фильтрация, остаются ограниченными в своей применимости для крупномасштабных применений. Сконструированные системы очистки водно-болотных угодий (CWTS) представляют собой энергосберегающие, экономически эффективные и устойчивые системы очистки воды, которые основаны на использовании растений и связанных с ними микробов для снижения содержания загрязняющих веществ в сточных водах; они стали многообещающей альтернативой для лечения OSPW 7,8,9,10,11,12.
CWTS — это спроектированные водно-болотные угодья, предназначенные для воспроизведения фильтрующих функций естественных водно-болотных угодий. Первоначально разработанные для очистки ливневых и муниципальных сточных вод, CWT в настоящее время используются для широкого спектра применений, включая сельскохозяйственные отходы, дренаж кислотных шахт, промышленные сточные воды и другие восстановительные работы13. Эти системы состоят из трех основных компонентов: субстрат, вода и растительность. CWTS может быть спроектирована как системы поверхностного или подземного потока, при этом движение воды настроено на горизонтальное или вертикальное течение13,14. Гидрофитные растения водно-болотных угодий широко используются в CWTS из-за их адаптации к постоянно насыщенным почвенным условиям. В целом, CWTS обычно использует новые виды растений, такие как Typha sp. (рогоз), Juncus sp. (камыш) и Carex sp. (осока).
В CWTS используются различные механизмы очистки воды. Взвешенные твердые частицы могут адсорбировать загрязняющие вещества и оседать, образуя осадочный слой, способствующий росту растений. Кроме того, растения могут переносить или преобразовывать растворенные загрязняющие вещества с помощью комбинации биотических и абиотических механизмов. Абиотические механизмы включают фильтрацию, осаждение, осаждение, сорбцию, химическое окисление/восстановление, комплексообразование, фотодеградацию и испарение. Биотические процессы включают биотрансформацию (микробную или растительную), фитоаккумуляцию и фитостабилизацию13,14. CWTS обладают значительными преимуществами в качестве самоподдерживающихся систем, которые, как правило, со временем становятся более эффективными14. Эти системы универсальны и способны обрабатывать несколько загрязняющих веществ одновременно, оставаясь при этом экологически устойчивыми и приемлемыми для общества. Кроме того, их низкие эксплуатационные и капитальные затраты по сравнению с традиционными методами очистки делают их хорошо подходящими для обработки больших объемов сточных вод, таких как OSPW. Тем не менее, сложность различных абиотических и биотических процессов, происходящих одновременно при OSPW, требует тщательного проектирования для оптимизации CWTS для достижения максимальной эффективности лечения. Четкое понимание целей лечения в сочетании с систематическим тестированием на лабораторном стенде, в пилотных и демонстрационных масштабах имеет важное значение для оптимизации системы и прогнозирования успеха полномасштабноговнедрения.
Эксперименты пилотного масштаба, часто называемые экспериментами с мезокосмом, обычно проводятся с использованием ванн или резервуаров, которые имитируют отдельные клетки для обработки. Мезокосмы могут проводиться как в помещении, так и на открытом воздухе в качестве полевого эксперимента. Мезокомы представляют собой частично закрытые системы, которые обеспечивают большую экологическую сложность, чем лабораторные эксперименты, сохраняя при этом достаточный контроль и воспроизведение для оценки влияния отдельных параметров конструкции на удаление загрязняющих веществ. Исследования в масштабе мезокосма необходимы для подтверждения механизмов лечения и выявления осложнений в меньшем масштабе, где могут быть реализованы коррекции и корректировки дизайна14. Этот протокол описывает настройку и функционирование горизонтального поверхностного потока в помещении в масштабе мезокосма, обеспечивая практическую основу для разработки исследований CWTS, особенно для ослабления NAFC в OSPW.
протокол
1. Построение мезокосма
ПРИМЕЧАНИЕ: Смотрите Таблицу материалов для полного списка материалов, необходимых для построения мезокосма, и Рисунок 1 для схемы построения мезокосма.
- При необходимости снимите верхнюю часть полиэтиленового бака (129,5 см x 30,0 см).
- Подготовить дренажные отверстия; Просверлите два отверстия (Детали #1 и #2) на одной и той же стороне полипропиленового бака. Вставьте фитинг для перегородки из ПВХ (Часть #3) в оба отверстия наружной резьбой наружу. Загерметизируйте внешний край штуцера переборки водостойким герметиком.
- Отверстие для отвода грунтовых вод (Часть #2): расположите его у углового основания бака, убедившись, что там достаточно места для крепления переборки.
- Отверстие для дренажа поверхностных вод (Часть #1): разместите его выше уровня почвы, ближе к центру резервуара.
- Поместите шайбу для шланга (Часть #4) с фильтрующей сеткой (Часть #5) на внутреннюю часть фитинга переборки и закрепите ее герметиком.
- Обустройте внутренний дренаж сантехники:
- Для отверстия для отвода поверхностных вод (Часть #1) сначала прикрепите адаптер с наружной резьбой из ПВХ (Часть #10) к фитингу перегородки (Часть #3), а затем колено из ПВХ под углом 90° (Часть #11).
- Вставьте кусок трубы ПВХ (Деталь #12), отрезанный так, чтобы высота желаемого уровня воды соответствовала колену 90°.
- Установите наружный дренажный водопровод. На следующих этапах используйте обжимные кольца для крепления PEX к фитингам.
- Оберните тефлоновую ленту вокруг резьбы 3/4-дюймового латунного адаптера PEX x 3/4-дюймового MPT (Часть #6) и подсоедините к фитингам перегородки (Часть #3).
- Вырежьте два равных отрезка 3/4 дюйма PEX (Часть #7) и прикрепите к латунным адаптерам MPT (Часть #6).
- Добавьте пластиковый расширительный угловой фитинг к трубе PEX (Часть #7), обращенным вниз для отверстия для дренажа поверхностных вод и обращенным к центру резервуара для дренажного отверстия для почвы.
- Для дренажного отверстия для почвы (Часть #2) подсоедините трубу PEX к колену, за которой следует шаровой кран, еще один сегмент PEX и пластиковый расширительный тройник. Отрегулируйте длину PEX, чтобы выровнять верхнюю часть расширительного тройника с водопроводом поверхностного дренажа.
- Для отверстия для отвода поверхностных вод подсоедините трубу PEX к пластиковому расширительному колену, соединив его с расширительным тройником.
- После того, как система будет подключена, добавьте еще один кусок PEX (Часть #7) к пластиковому расширительному тройнику, заканчивающемуся пластиковым расширительным коленом, обращенным вниз.
- Добавьте еще один кусок PEX (Часть #7) на дно пластикового расширительного колена, чтобы вода стекала в резервуар резервуара.
- Повысить структурную целостность мезокосма:
- Соберите каркас (Деталь #13, длина 129,5 см x ширина 37,0 см) из кусков пиломатериалов размером 2 x 4 дюйма.
- Закрепите каркас шурупами по дереву.
- Поместите раму на мезокосм, следя за тем, чтобы она не сидела на сантехнической арматуре.
- Оберните внешнюю сторону мезокосма алюминиевой фольгой, чтобы уменьшить попадание света в почву со стороны мезокосма.
2. Настройка и обслуживание мезокосма
- Выращивают растения для эксперимента из семян:
- По мере необходимости стратифицировать семена.
- Поместите семена в стандартные контейнеры из стироблока, содержащие торф в качестве пробки.
- Как только рассада прорастет, удобряйте ее 3 раза в неделю, используя водорастворимую подкормку для растений (24-8-16).
- Дайте рассаде вырасти минимум 3-5 месяцев, чтобы убедиться, что она достигнет оптимального размера для лечения и ответа.
ПРИМЕЧАНИЕ: Точная продолжительность времени будет зависеть от размера и типа вида. Этот шаг можно пропустить, если рассада приобретается, а не выращивается.
- Поместите мезокосмы в теплицу:
- (Дополнительный) Укрепите тепличные столы фанерой, чтобы выдержать вес мезокосмов.
- Равномерно распределите мезокосмы по столам парниковых отсеков, чтобы обеспечить случайное размещение обработок и свести к минимуму изменения условий окружающей среды (рис. 2).
- Расположите сантехнику так, чтобы она свисала с края стола для надлежащего дренажа в резервуар (рис. 2).
- Настройте резервуарный бак:
- Поместите пластиковый промышленный барабан с открытым верхом объемом 57 л под дренажную трубу.
- Установите погружной циркуляционный насос Powerhead между средней и нижней частями резервуара, чтобы обеспечить непрерывное смешивание в резервуаре. Закрепите шнур питания снаружи бака.
- Добавьте и пропитайте субстрат:
- Равномерно распределите субстрат в мезокосме и утрамбуйте субстрат с умеренным давлением до нужной высоты.
ПРИМЕЧАНИЕ: Высота субстрата зависит от целей исследования и вида растений. - Полностью насытить субстрат водой обратного осмоса (ОО), измерить объем добавленной воды; Это эквивалентно объему поровой воды в субстрате.
ПРИМЕЧАНИЕ: Поровая вода - это объем воды, добавленный при насыщении субстрата, что можно наблюдать, когда уровень воды соответствует верху субстрата. Этот процесс может занять до суток. Объем поровой воды важен для определения точного количества воды в системе и расчета расхода.
- Равномерно распределите субстрат в мезокосме и утрамбуйте субстрат с умеренным давлением до нужной высоты.
- Определите расход:
- Выберите время хранения на основе предыдущих исследований и целей исследования.
- Рассчитайте общий объем воды в мезокосме.

- Рассчитайте расход.
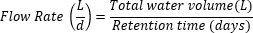
- Установите насосы:
- Расположите один насос между двумя соседними мезокосмами.
ПРИМЕЧАНИЕ: При необходимости один насос также может быть использован для одного мезокосма. - Соедините все насосы вместе с помощью USB-кабеля типа «вилка-вилка», подключив последний насос к контроллеру.
- Погрузите трубку с клапаном в резервуар, закрепив или утяжелив ее, чтобы она оставалась на месте.
- Закрепите трубку выпускного клапана в заднем верхнем углу мезокосма, следя за тем, чтобы она оставалась выше ватерлинии.
- Оберните трубку алюминиевой фольгой, чтобы предотвратить рост водорослей.
- Настройте и откалибруйте насосы, блок питания и контроллер в соответствии с инструкциями производителя15.
- Отрегулируйте насосы в соответствии с расчетным расходом.
- Расположите один насос между двумя соседними мезокосмами.
- Посадите и акклиматизируйте виды растения:
- Отрегулируйте температуру и светодиодные лампы для выращивания растений до оптимального уровня для роста растений, одновременно приспосабливая виды растений к мезокосме.
- Равномерно посадите 6-12 отдельных видов растений, чтобы обеспечить одинаковую биомассу на единицу площади в мезокосме.
Примечание: Количество особей может меняться в зависимости от целей исследования и физиологии вида (например, по мере того, как Typha latifolia становится корневым, количество особей может уменьшаться). - Постепенно повышайте уровень воды обратного осмоса, поддерживая один уровень воды в течение 1-2 дней, и заменяйте трубу из ПВХ (шаг 1.4.2) по мере необходимости, чтобы она соответствовала уровню воды.
- Включите насосы с конечным нужным расходом.
- Как только желаемый уровень воды будет достигнут, отрегулируйте освещение и температуру в теплице до экспериментальных параметров и дайте растениям акклиматизироваться в течение ~35 дней.
- Слейте воду и промойте систему:
- Снимите стояк из ПВХ и откройте шаровой кран, чтобы полностью слить воду из системы; Это может занять до 2 дней.
- Промойте систему с помощью OSPW и дайте ей полностью стечь, убедившись, что труба из ПВХ остается выключенной, а шаровой кран открытым. Убедитесь, что OSPW, использованный во время промывки, не используется во время эксперимента.
- После промывки закройте шаровой кран и добавьте трубу из ПВХ в соответствии с желаемым уровнем воды.
- Добавьте OSPW:
- Осторожно вливайте OSPW в каждый мезокосм, чтобы не потревожить субстрат или растения, заполняя до тех пор, пока не будет достигнут нужный уровень воды.
- При использовании нескольких партий воды убедитесь, что химические свойства стабильны, или равномерно распределите по всем мезокосмам.
- Заполните бак резервуара OSPW, оставив примерно 5 см пространства сверху.
- Управление испарением:
- При необходимости наполняйте бак резервуара водой обратного осмоса, поддерживая уровень воды примерно на 5 см ниже верха.
3. Отбор проб
- Видовые измерения растений:
- Каждый временной цикл удержания измеряйте показатели здоровья и роста растений16. Показатели здоровья растений включают видимые признаки стресса, такие как хлороз и повреждение насекомыми, в то время как показатели роста растений включают смертность, высоту и % покрытия.
- В конце эксперимента возьмите пробы на надземную биомассу растений и химический состав растительных тканей, если это необходимо.
ПРИМЕЧАНИЕ: Используемые интервалы мониторинга и измерения являются рекомендуемыми для изучения влияния НАФК на здоровье растений и могут отличаться в зависимости от целей эксперимента.
- Размеры основания:
- Базовая характеристика: Прежде чем добавлять субстраты в каждый мезокосм, измерьте набор параметров (например, pH, электропроводность (EC), окислительно-восстановительный потенциал (ОВП), основные анионы/катионы, питательные вещества, NAFC и любые другие соответствующие загрязнители).
- Во время первого цикла удержания соберите образцы субстрата из каждого мезокосма, чтобы получить исходный уровень для общей химии. Соберите образцы субстрата из случайных мест в каждом мезокосме.
- В каждом временном цикле удержания измеряйте ОВП подложки с помощью соответствующего зонда ОВП.
- В конце эксперимента соберите образцы субстрата из каждого мезокосма и измерьте те же параметры, что и при базовой характеристике (например, pH, EC, ОВП, основные анионы/катионы, питательные вещества, NAFC и любые другие соответствующие загрязнители).
- Измерения воды:
- Базовая характеристика: перед добавлением OSPW в каждый мезокосм измерьте набор параметров (например, pH, EC, ОВП, основные анионы/катионы, питательные вещества, NAFC и любые другие соответствующие загрязнители).
- После начала эксперимента через несколько дней (в конце цикла удержания 1) возьмите первоначальные пробы OSPW из каждого мезокосма, чтобы осадок в OSPW остыл и чтобы OSPW заполнил пористое водное пространство. Соберите образцы OSPW с лицевой стороны каждого мезокосма.
- В каждом временном цикле удержания измеряйте концентрацию растворенного кислорода (РК), ОВП, pH, EC и температуры с помощью эталонного прибора.
- В конце эксперимента соберите окончательные пробы воды для измерения общего химического состава, измерьте набор параметров (например, DO, PH, EC, ОВП, основные анионы/катионы, питательные вещества, NAFC и любые другие соответствующие загрязнители).

Рисунок 1: Схема конструкции мезокосма и экспериментальной установки. (А) Схема конструкции мезокосма и необходимых компонентов. (B) Пример экспериментальной установки, включая добавление субстрата и растений, а также размещение резервуара. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.

Рисунок 2: Пример расположения мезокосма и водохранилища. (А) Раскладка мезокосмов и резервуарных баков в теплице без алюминиевой фольги. (B) Схема, показывающая мезокосмы и резервуарные резервуары с алюминиевой фольгой, обернутые вокруг мезокосмов, для ограничения проникновения света, с одним насосом на два мезокосма. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
Результаты
Успех этого протокола по водно-болотным угодьям демонстрируется устойчивым ростом и развитием видов растений, постоянным мониторингом параметров окружающей среды и эффективным удалением загрязняющих веществ с течением времени. Данные, собранные Trepanier et al.17 , иллюстрируют эффективность метода и ожидаемые результаты. В исследовании оценивалась способность Carex aquatilis, водяной осоки, обычно встречающейся в бореальных водно-болотных угодьях, снижать NAFC в OSPW. В нем сравнивались показатели мезокосмов с C. aquatilis и без растений, использующих либо OSPW, либо лабораторную технологическую воду. Мезокосмы были построены из субстрата из 10 см крупнозернистых песчаных хвостов (CST), покрытых 10 см минеральной торфяной смеси (PMM) и 25 см OSPW, покрывающих основания. Перед экспериментом растения выращивали в течение 3 месяцев в среднем до высоты 83 см, а затем пересаживали в систему. Для акклиматизации растений к мезокосму добавляли воду обратного осмоса (рис. 3), и системы поддерживали при температуре 20 °C в течение 32 дней.

Рисунок 3: Виды посадки и добавление воды обратного осмоса. (А) Добавление в субстрат утрамбованного субстрата и примера посадочных видов. (Б) Равномерное распределение видов растений по всему мезокосме. (В) Добавление воды обратного осмоса в мезокосмы на период акклиматизации растений. Аббревиатура: RO = обратный осмос. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
Растения демонстрировали устойчивый рост на протяжении всего эксперимента, с заметным увеличением высоты и покрова (Рисунок 4). Рисунок 5 также иллюстрирует устойчивый рост C. aquatilis, достигающий высоты примерно 150 см к 40-му дню до выхода на плато. Это было в пределах ожидаемого диапазона роста C. aquatilis в 20-155 см. Выживаемость растений была высокой и составила 98%, при этом к концу эксперимента 99% сохранялись в живых тканях растений. Тем не менее, у большинства растений наблюдались признаки хлороза, некроза и/или крапчатости, а в некоторых случаях деформированные и морщинистые листья17. Регулярный мониторинг здоровья растений имеет жизненно важное значение для выявления потенциальных проблем, таких как заражение вредителями.

Рисунок 4: Фотографии роста растений в начале и в конце эксперимента. Пример фото роста и здоровья Carex aquatilis с 0 по 78 день. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
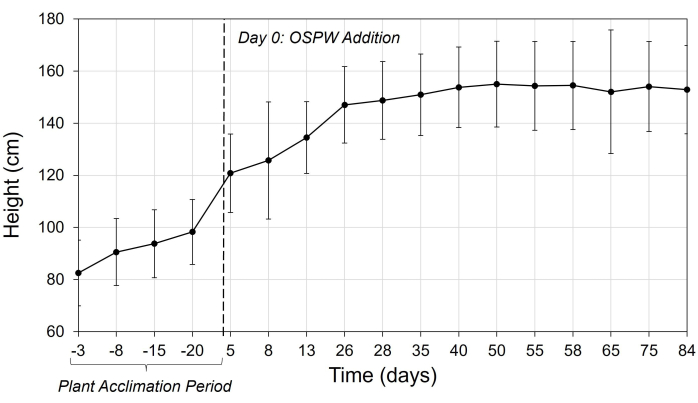
Рисунок 5: Высота растения с течением времени в мезокосме, содержащем Carex aquatilis. Средняя высота растений для Carex aquatilis в мезокосмах (n = 48). День 0 — это время, когда OSPW был добавлен в систему. Период акклиматизации растений относится к периоду, когда мезокосмы содержали воду обратного осмоса до добавления OSPW. Полосы погрешностей указывают на одно стандартное отклонение от среднего значения. Этот рисунок был адаптирован из Trepanier et al.17. Сокращения: RO = обратный осмос; OSPW = вода из нефтеносных песков, подверженная технологическому воздействию. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
Ключевые параметры окружающей среды, такие как растворенный раствор воды и окислительно-восстановительный потенциал субстрата, регулярно контролировались для обеспечения оптимальной производительности системы, поскольку поддержание достаточного уровня кислорода имеет решающее значение для здоровья растений и эффективного удаления загрязняющих веществ в CWTS. Окислительно-восстановительные значения субстрата колебались на протяжении всего эксперимента, при этом непосаженные мезокосмы оставались в окислительных условиях от 50 мВ до 100 мВ, в то время как мезокосмы, содержащие C. aquatilis , иногда приближались к 0 мВ. OSPW поддерживал уровень РК > 5 ppm на протяжении всего эксперимента, и РК был выше в целом при мезокосмах без растений, особенно к концу эксперимента (рис. 6). РК 8 ppm часто считается идеальным для роста растений; однако допустимо значение РК выше 5 ppm. Регулярный мониторинг позволяет выявлять периодические снижения растворенного кислорода, что может повлечь за собой проведение проверок системы, таких как проверка функциональности насоса, для обеспечения стабильной работы.
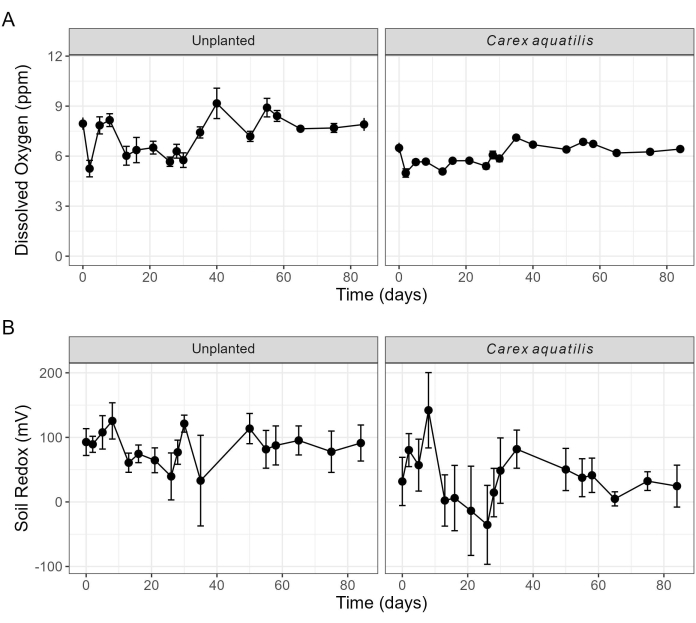
Рисунок 6: Измерения растворенного кислорода и окислительно-восстановительного потенциала почвы в мезокосмах. (A) Растворенный кислород в OSPW и (B) потенциал почвенного окислительно-восстановительного потенциала для мезокосмов с Carex aquatilis и непосаженных обработок только с помощью OSPW. Точки данных представляют собой средние значения из четырех повторяющихся мезокосмов (n = 4), где полосы погрешностей указывают на одну стандартную ошибку среднего. Аббревиатура: OSPW = вода, пострадавшая от технологических процессов нефтеносных песков. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
Основной целью исследования была оценка потенциала ослабления NAFC от OSPW с использованием мезокосма CWTS. На рисунке 7 показано постепенное снижение концентраций NAFC на протяжении всего эксперимента, что демонстрирует эффективность системы. Присутствие C. aquatilis усиливало удаление NAFC, достигая снижения NAFC на 76% в течение 82 дней (72,1 мг/л в начале до 17,1 мг/л в конечную конечную дозу) по сравнению с 8,5% в непосаженной контрольной обработке в течение 82 дней (64,5 мг/л от начальной до 59,0 мг/л в конечную)17. Успешное снижение концентрации NAFC, наряду со здоровым ростом растений и благоприятными условиями окружающей среды, подтверждают, что мезокосм работает эффективно. Эти результаты демонстрируют способность системы моделировать искусственные водно-болотные угодья и дают ценную информацию о роли CWTS в снижении токсичности OSPW.

Рисунок 7: Концентрация NAFC во времени в мезокосмах. Концентрация соединений фракции нафтеновой кислоты в мезокосмах с Carex aquatilis и непосаженных обработках только OSPW. Точки данных представляют собой средние значения из четырех повторяющихся мезокосмов (n = 4), где полосы погрешностей указывают на одну стандартную ошибку среднего. Разные буквы между средними указывают на существенную разницу (P < 0,05). Этот рисунок был адаптирован из Trepanier et al.17. Сокращения: OSPW = вода, подверженная технологическому воздействию нефтеносных песков; NAFC = соединения фракции нафтеновой кислоты. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
Обсуждение
CWTS используются в качестве пассивной и экономически эффективной очистки для многих сточных вод13; тем не менее, они являются относительно новым методом лечения OSPW для ослабления NAFC 7,8,9,10,11,12,17,18. Используя методы, описанные в данной статье, эффективность CWTS может быть повышена за счет оценки различных параметров конструкции.
Мезокомы собираются, как показано на рисунке 1, обеспечивая правильную установку дренажных трубопроводов. Чтобы предотвратить возможные проблемы с потоком или неравномерное время удержания, вызванные засорением выпускных отверстий основанием, на нижней дренажной пробке размещается шланговая мойка с фильтрующим экраном, а верхнее дренажное отверстие располагается над уровнем основания. Если засоры возникают, несмотря на эти меры, для устранения засоров можно использовать дренажный шнек или давление воздуха.
Мезокосмы размещаются на тепличных столах, укрепленных фанерой, с ведрами-резервуарами, расположенными на концах столов для рециркуляции воды. Вода циркулирует по системе с помощью самотека, входя через наливной шланг и выходя в конце поверхностного дренажного отверстия, прежде чем вернуться в резервуар. Время удержания (дни) было выбрано на основе ранее построенных исследований водно-болотных угодий7. Погружные циркуляционные насосы используются для обеспечения непрерывного перемешивания пласта. Дозирующие насосы используются для облегчения перемещения воды между мезокосмом и резервуаром. Есть возможность подключения одного дозирующего насоса к двум мезокосмам. Насосы должны быть настроены на основе экспериментальных целей для достижения желаемого расхода и времени удержания.
После построения мезокосма субстрат равномерно уплотняется в мезокосмах, растения пересаживаются, а также добавляется вода обратного осмоса. Вода обратного осмоса используется первоначально в период акклиматизации растений, чтобы обеспечить хорошее функционирование системы со здоровыми растениями до начала эксперимента. После периода акклиматизации мезокосмы дренируют, промывают 100% OSPW в течение 24 ч для обеспечения замены поровой воды, а затем снова заполняют OSPW перед началом эксперимента.
Ключевые измерения, которые должны быть выполнены, включают показатели здоровья и роста растений, параметры субстрата и химического состава воды, а также концентрации целевого загрязнителя. Рутинные измерения параметров воды и субстрата проводятся один раз за цикл, чтобы убедиться, что мезокосм работает должным образом. Рекомендуется измерять параметры качества воды, включая растворенный раствор, ОВП, pH и проводимость, один раз за цикл с помощью многопараметрического прибора YSI Professional Plus. ОВП почвы и РК в воде являются ключевыми параметрами, которые необходимо контролировать, чтобы обеспечить поддержание аэробных условий мезокосмами.
Описанный метод обладает высокой адаптивностью и может быть изменен в зависимости от целей лечения. Основные модификации обработки включают, помимо прочего, виды растений, использование нескольких видов растений, время хранения, условия окружающей среды, состав и глубину субстрата, а также добавление удобрений. Виды растений следует выбирать на основе характеристик, повышающих выживаемость растений и эффективность фиторемедиации. Выбор местных видов растений водно-болотных угодий, адаптированных к местному климату, повысит вероятность успешного роста и выживания 11,13,14. К видам растений, которые хорошо подходят для использования в CWT, относятся те, которые развивают глубокие и широкие корни, сильные корневища, быстрый рост, достаточный транспорт кислорода и обладают механизмами противодействия эффектам солености 17,19,20. Часто рекомендуется избегать посадки смесей видов растений, так как увеличение разнообразия растений может привести к снижению уверенности в эффективности CWTS. Особенно если одно растение становится доминирующим, трудно смоделировать, как будут вести себя CWTS14. Выбранные виды растений также влияют на эвапотранспирацию, которая может оказывать эффект концентрации соли и других загрязняющих веществ.
Важно обеспечить учет эвапотранспирации в системе; обеспечение поддержания уровня OSPW с помощью воды обратного осмоса. Использование муниципальной воды или воды, не относящейся к обратному осмосу, может привести к увеличению концентрации других компонентов (например, хлорида, кальция, фтора), что может повлиять на результаты исследования мезокосма. Изменение времени удержания может помочь с аэрацией, гарантируя, что различные компоненты и уровни в мезокосме не станут анаэробными, что может привести к воздействию на микробные сообщества и здоровье растений.
Импульсные или прерывистые притоки могут использоваться для моделирования естественной динамики водно-болотных угодий (т.е. штормовых явлений и сезонного стока). Обеспечение того, чтобы переменные окружающей среды (температура, условия освещенности и сезонные колебания) были аналогичны переменным в исследуемой области, важно для экстраполяции работы на крупномасштабные CWTS, поскольку это уменьшит количество новых переменных, которые будут влиять на систему, и анализ того, как эти переменные влияют на эффективность CWTS в ослаблении NAFC. Выбор субстратов для мезокосмов, которые можно использовать на более масштабных CWTS, поможет информировать о будущем дизайне и повысить эффективность системы очистки. При добыче нефтеносных песков крупнозернистые песчаные хвосты и торфяно-минеральная смесь являются субстратами и ранее были протестированы в исследованиях мезокосма для определения оптимального субстрата для улучшения здоровья растений, увеличения полезных микробных сообществ и помощи в ослаблении NAFCs17.
Основным ограничением этого метода является ограниченный размер и глубина мезокосма, что может повлиять на рост корней и привести к тому, что растения станут корневыми. Эти ограничения могут быть преодолены путем сокращения продолжительности эксперимента и/или количества используемых отдельных растений. Если в одном и том же мезокосме используется несколько веществ, могут возникнуть синергетические или аддитивные эффекты от конкуренции. В конечном счете, размер и глубина мезокосма могут привести к сокращению продолжительности эксперимента, ограничивая объем собираемых данных. В более долгосрочных экспериментах можно изучать такие процессы, как круговорот питательных веществ, которые происходят, когда органические вещества добавляются в систему путем накопления и медленного разложения растительного детрита и корневых выделений. Это может повлиять на микробные сообщества и скорость затухания загрязняющих веществ. Кроме того, относительно короткие экспериментальные временные рамки этого дизайна мезокосма обеспечивают быструю обратную связь, которая может быть использована для улучшения будущих экспериментов. Питательные вещества могут быть добавлены в систему мезокосма; Тем не менее, тип и количество добавляемых удобрений требуют тщательного мониторинга для предотвращения цветения водорослей.
Условия в теплице настроены таким образом, чтобы создать оптимальные условия для выращивания; Температурные диапазоны установлены таким образом, чтобы они надлежащим образом отражали сезонные температуры региона, с постепенными изменениями, осуществляемыми для моделирования естественных суточных колебаний. Уровень влажности также регулируется в диапазоне, репрезентативном для регионального климата. Кроме того, теплица рассчитана на получение 25 000 люкс, что эквивалентно примерно 200 Вт/м² окружающего дневного света, в течение назначенного светового дня. Чтобы обеспечить постоянную интенсивность света, светодиодные лампы активируются, когда уровень естественного освещения падает ниже этого порога. Использование теплицы также имеет свои ограничения. Несмотря на то, что теплицы обеспечивают контролируемую среду, они также могут создавать уникальные проблемы, такие как заражение вредителями, парниковые эффекты и создание неестественной среды. Заражение вредителями особенно распространено в тепличных условиях и может повлиять на здоровье и рост растений. Чтобы сократить использование инсектицидов, отличными альтернативами являются естественные хищники или физическое удаление вредителей. Несмотря на эти проблемы, теплица остается оптимальной средой для проведения пилотного исследования, поскольку она позволяет точно контролировать и изучать отдельные параметры.
Этот метод представляет собой один из многих подходов к планированию экспериментов по мезокосму. Опытно-промышленные эксперименты CWTS могут проводиться как на открытом воздухе10,21, так и в помещении 4,17. На мезокосмы на открытом воздухе влияют многомерные факторы окружающей среды, которые могут взаимодействовать сложным и непредсказуемым образом. Эти взаимодействия затрудняют моделирование отдельных переменных или выяснение конкретных механизмов, влияющих на наблюдаемые результаты. В результате становится трудно определить, какие факторы влияют на производительность CWTS, и выявить возможности для улучшения проектирования системы; однако они более точно воспроизводят полномасштабные условия CWTS14. В отличие от этого, мезокосмы внутри помещений обеспечивают более контролируемую среду, сводя к минимуму воздействие природы и других внешних воздействий, облегчая понимание процессов и определение проектных параметров, которые могут повысить производительность.
В конструкциях CWTS обычно используется либо горизонтальный поверхностный поток 4,10,17,18, либо вертикальный подземный поток18. Описанный здесь метод представляет собой проектирование горизонтального поверхностного потока. В то время как системы вертикального потока полагаются на силу тяжести для облегчения вертикального движения воды, обеспечивая лучшее насыщение кислородом и требуя меньше места, системы горизонтального потока поддерживают более стабильные условия10 и увеличивают потенциал фиторемедиации22. Мезокосмы предлагают значительные преимущества для разработки CWTS за счет тестирования интегральных компонентов и повышения эффективности для будущих крупномасштабных применений, обеспечивая воспроизводимость и контроль окружающей среды, а также позволяя выделять и измерять отдельные экспериментальные параметры, а также отслеживать биотические изменения и пути рассеивания химических веществ.
Раскрытие информации
У авторов нет конфликта интересов, который можно было бы раскрыть.
Благодарности
Финансирование этого исследования было предоставлено Канадским проектом крупномасштабных прикладных исследований Genome (LSARP, грант #18207) и программой финансирования Канадской лесной службы «Кумулятивные эффекты». Мы хотели бы поблагодарить компанию Imperial Oil Ltd. за предоставление материалов, использованных в этом исследовании. Мы также хотели бы поблагодарить всех, кто помогал в экспериментах: Яна Вандер Мёлена, Джейсона М.Е. Ахада, Сару Корреа-Гарсия, Саймона Морвана, Мари-Жозе Бержерон, Дилини Атугалу, Лизу Гиг, Джона В. Хедли, Этьена Ерго и Кристин Мартино. Мы также хотели бы поблагодарить Дугласа Мюнха за экспериментальный и мезокосмический дизайн. Мы также хотели бы поблагодарить сотрудников Северного лесного центра и летних студентов, которые помогали на протяжении всех экспериментов. Мы хотели бы отметить, что наши исследования проводились на территории Договора No 6, а материалы, источники для этих экспериментов были собраны на территории Договора No 8. Мы признаем и чтим коренные народы, метисов и инуитов, которые жили, собирались и путешествовали на этих землях.
Материалы
| Name | Company | Catalog Number | Comments |
| 2-inch x 4-inch x 12 ft Lumber | Any Supplier | N/A | |
| 3/4-inch Brass PEX Ball Valve | Any Supplier | N/A | |
| 3/4-inch Copper PEX Crimp Ring for PEX Pipe | Any Supplier | N/A | |
| 3/4-inch IPEX Schedule 40 PVC 90° Welding Street Elbow | Any Supplier | N/A | |
| 3/4-inch PEX Stick White | Any Supplier | N/A | For the outside of the mesocosm |
| 3/4-inch PEX x 3/4-inch MPT Brass adapter | Any Supplier | N/A | |
| 3/4-inch Plastic Expansion Elbow Fitting for PEX | Any Supplier | N/A | |
| 3/4-inch Plastic Expansion Tee for PEX | Any Supplier | N/A | |
| 3/4-inch PVC Bulkhead Fitting Water Tank Connector Adapter | Any Supplier | N/A | |
| 3/4-inch PVC Schedule 40 / 90 Degree Elbow | Any Supplier | N/A | |
| 3/4-inch PVC Schedule 40 Male Adapter | Any Supplier | N/A | |
| 3/4-inch PVC White | Any Supplier | N/A | For the inside of the mesocosm |
| 4-inch Wood Screws | Any Supplier | N/A | |
| Aluminum Foil | Any Supplier | N/A | |
| Aquarium Submersible Powerhead Circulation Pump | Any Supplier | N/A | Suction cup or magnetic |
| Hose Washer | Any Supplier | N/A | |
| Miracle Grow water-soluble plant food | Miracle Grow | N/A | 24-8-16 formula |
| Neptune Apex A3 Aquarium Controller and Power Bar | Neptune Systems | N/A | |
| Neptune Apex DOS Quiet Drive Dosing Pump | Neptune Systems | N/A | |
| Neptune AquaBus Cable - 15-Foot Male/Male | Neptune Systems | N/A | |
| Neptune DOS DDR Tubing | Neptune Systems | N/A | |
| Open Top Plastic Industrial Drum | Any Supplier | N/A | 57 L |
| Petri dish | Any Supplier | N/A | For seed stratication |
| Peat | Any Supplier | N/A | |
| Polypropylene Tank | D&M Plastics Inc. | RW1016 | 50.8 cm height × 33.0 cm width × 129.5 cm length; 248.1 L |
| Silicone All-Purpose Waterproof Sealant (Aquarium Grade) | Any Supplier | N/A | |
| Standard styroblock containers (415A) | Any Supplier | N/A | |
| Teflon Tape | Any Supplier | N/A | |
| YSI Professional Plus Multiparameter instrument | YSI Inc. | 6050000 |
Ссылки
- Alberta Geological Survey Oil Sands. , Alberta Geological Survey. https://ags.aer.ca/our-science/oil-and-gas/oil-sands (2024).
- Alberta Geological Survey. Energy Regulator State of Fluid Tailings Management for Mineable Oil Sands, 2020. , 83(2021).
- Allen, E. W. Process water treatment in Canada's oil sands industry: II. A review of emerging technologies. J Environ Eng Sci. 7 (5), 499-524 (2008).
- McQueen, A. D., et al. Performance of a hybrid pilot-scale constructed wetland system for treating oil sands process-affected water from the Athabasca oil sands. Ecol Eng. 102, 152-165 (2017).
- Hughes, S. A., et al. Using ultrahigh-resolution mass spectrometry and toxicity identification techniques to characterize the toxicity of oil sands process-affected water: The case for classical naphthenic acids. Environ Toxicol Chem. 36 (11), 3148-3157 (2017).
- Morandi, G. D., et al. Effects-directed analysis of dissolved organic compounds in oil sands process-affected water. Environ Sci Technol. 49 (20), 12395-12404 (2015).
- Ajaero, C., et al. Fate and behavior of oil sands naphthenic acids in a pilot-scale treatment wetland as characterized by negative-ion electrospray ionization Orbitrap mass spectrometry. Sci Total Environ. 631 - 632, 829-839 (2018).
- Ajaero, C., et al. Developments in molecular level characterization of naphthenic acid fraction compounds degradation in a constructed wetland treatment system. Environments. 7 (10), 1-16 (2020).
- Cancelli, A. M., Gobas, F. A. P. C. Treatment of naphthenic acids in oil sands process-affected waters with a surface flow treatment wetland: Mass removal, half-life, and toxicity-reduction. SSRN Electronic Journal. 213, 113755(2022).
- Cancelli, A. M., Gobas, F. A. P. C. Treatment of polycyclic aromatic hydrocarbons in oil sands process-affected water with a surface flow treatment wetland. Environments. 7 (9), 1-16 (2020).
- Cancelli, A. M., Borkenhagen, A. K., Bekele, A. A vegetation assessment of the Kearl treatment wetland following exposure to oil sands process-affected. Water. 14 (22), 1-18 (2022).
- Simair, M. C., et al. Treatment of oil sands process affected waters by constructed wetlands: Evaluation of designs and plant types. Sci Total Environ. 772, 145508(2021).
- Constructed Treatment Wetland. , Interstate Technology Regulatory Council Mining Waste Treatment Technology Selection. https://projects.itrcweb.org/miningwaste-guidance/to_const_treat.htm (2010).
- Haakensen, M., Pittet, V., Spacil, M. M., Castle, J. W., Rodgers, J. H. Jr Key aspects for successful design and implementation of passive water treatment systems. J Environ Solutions Oil Gas Mining. 1 (1), 59-81 (2015).
- Get started identifying the Apex and EB832. , Available from: https://help.neptunesystems.com/getstarted/apexng/ (2024).
- Pouliot, R., Rochefort, L., Graf, M. D. Impacts of oil sands process water on fen plants: Implications for plant selection in required reclamation projects. Environ Pollut. 167, 132-137 (2012).
- Trepanier, K. E., Vander Meulen, I. J., Ahad, J. M. E., Headley, J. V., Degenhardt, D. Evaluating the attenuation of naphthenic acids in constructed wetland mesocosms planted with Carex aquatilis. Environ Monit Assess. 195 (10), 1228(2023).
- Hendrikse, M., et al. Treatment of oil sands process-affected waters using a pilot-scale hybrid constructed wetland. Ecol Eng. 115, 45-57 (2018).
- Albert, R., Popp, M. Chemical composition of halophytes from the Neusiedler Lake region in Austria. Oecologia. 27 (2), 157-170 (1977).
- Cooper, A. The effects of salinity and waterlogging on the growth and cation up take of salt marsh plants. New Phytol. 90 (2), 263-275 (1982).
- Reis, P. C. J., et al. Microbial degradation of naphthenic acids using constructed wetland treatment systems: metabolic and genomic insights for improved bioremediation of process-affected water. FEMS Microbiol Ecol. 99 (12), fiad153(2023).
- Yang, L., Bekele, A., Gamal El-Din, M. Comprehensive characterization of organics in oil sands process water in constructed mesocosms utilizing multiple analytical methods. Environ Res. 252, 118972(2024).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеThis article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены