16.9 : Titration of a Polyprotic Acid
A polyprotic acid contains more than one ionizable hydrogen and undergoes a stepwise ionization process. If the acid dissociation constants of the ionizable protons differ sufficiently from each other, then the titration curve for such polyprotic acid generates a distinct equivalence point for each of its ionizable hydrogens. Therefore, titration of a diprotic acid results in the formation of two equivalence points, whereas the titration of a triprotic acid results in the formation of three equivalence points on the titration curve.
Carbonic acid, H2CO3, is an example of a weak diprotic acid. The first ionization of carbonic acid yields hydronium ions and bicarbonate ions in small amounts.
First ionization:
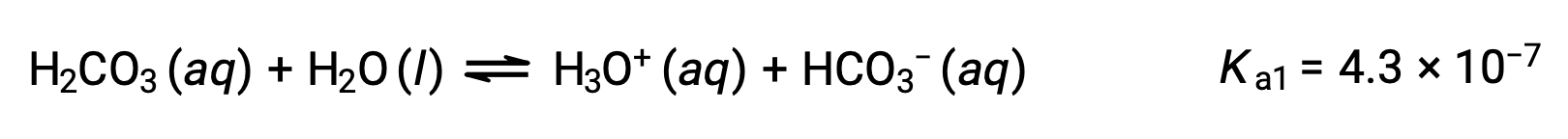
The bicarbonate ion can also act as an acid. It ionizes and forms hydronium ions and carbonate ions in even smaller quantities.
Second ionization:
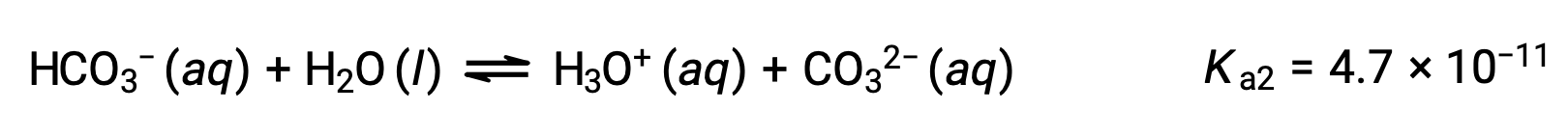
The Ka1 is larger than the Ka2 by a factor of 104. Therefore, when H2CO3 is titrated with a strong base like NaOH, it produces two distinct equivalence points for each ionizable hydrogen.
Phosphoric acid, a triprotic acid, ionizes in three steps:
First ionization:
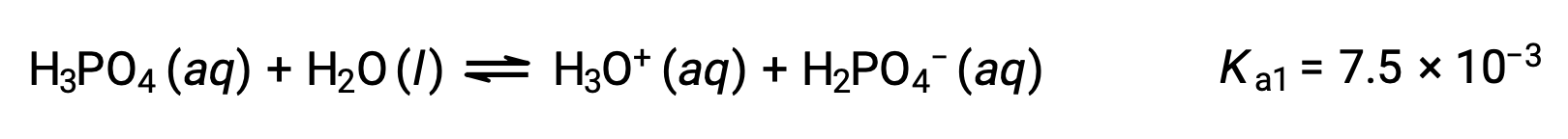
Second ionization:
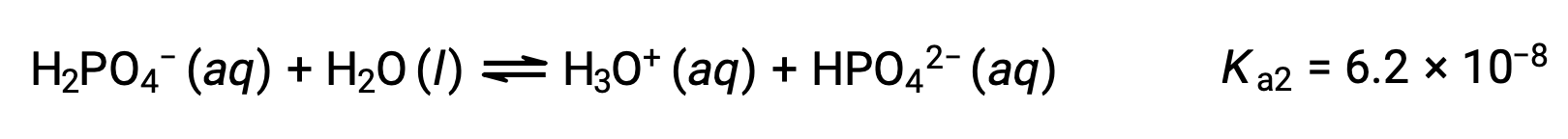
Third ionization:
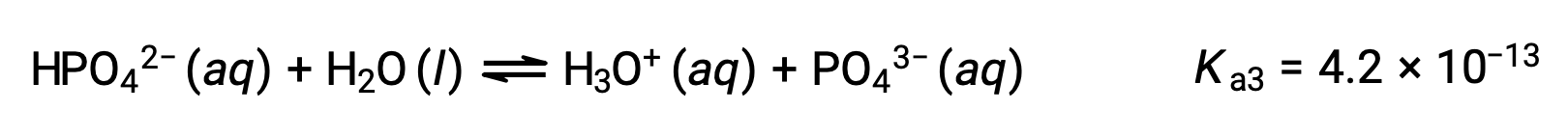
When H3PO4 is titrated with a strong base like KOH, it produces three equivalence points for each ionizable hydrogen. However, as HPO42− is a very weak acid, the third equivalence point is not easily discernible on the titration curve.
This text is adapted from Openstax, Chemistry 2e, Section 14.5: Polyprotic Acids.
From Chapter 16:

Now Playing
16.9 : Titration of a Polyprotic Acid
Acid-base and Solubility Equilibria
94.6K Views

16.1 : Common Ion Effect
Acid-base and Solubility Equilibria
39.9K Views

16.2 : Buffers
Acid-base and Solubility Equilibria
161.6K Views

16.3 : Henderson-Hasselbalch Equation
Acid-base and Solubility Equilibria
66.7K Views

16.4 : Calculating pH Changes in a Buffer Solution
Acid-base and Solubility Equilibria
50.9K Views

16.5 : Buffer Effectiveness
Acid-base and Solubility Equilibria
47.3K Views

16.6 : Titration Calculations: Strong Acid - Strong Base
Acid-base and Solubility Equilibria
27.8K Views

16.7 : Titration Calculations: Weak Acid - Strong Base
Acid-base and Solubility Equilibria
42.2K Views

16.8 : Indicators
Acid-base and Solubility Equilibria
46.9K Views

16.10 : Solubility Equilibria
Acid-base and Solubility Equilibria
49.4K Views

16.11 : Factors Affecting Solubility
Acid-base and Solubility Equilibria
32.4K Views

16.12 : Formation of Complex Ions
Acid-base and Solubility Equilibria
22.6K Views

16.13 : Precipitation of Ions
Acid-base and Solubility Equilibria
27.0K Views

16.14 : Qualitative Analysis
Acid-base and Solubility Equilibria
17.6K Views

16.15 : Acid-Base Titration Curves
Acid-base and Solubility Equilibria
123.4K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.
