16.7 : Titration Calculations: Weak Acid - Strong Base
Calculating pH for Titration Solutions: Weak Acid/Strong Base
For the titration of 25.00 mL of 0.100 M CH3CO2H with 0.100 M NaOH, the reaction can be represented as:
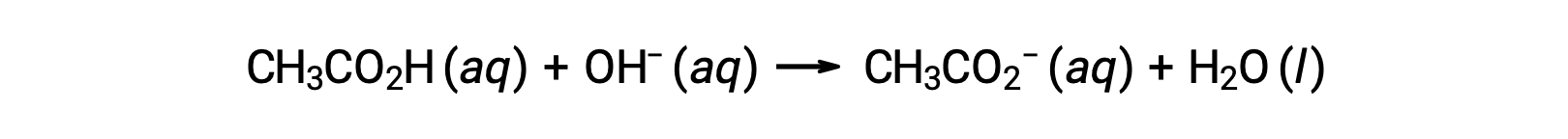
The pH of the titration solution after the addition of the different volumes of NaOH titrant can be calculated as follows:
(a) The initial pH is computed for the acetic acid solution in the usual ICE approach:
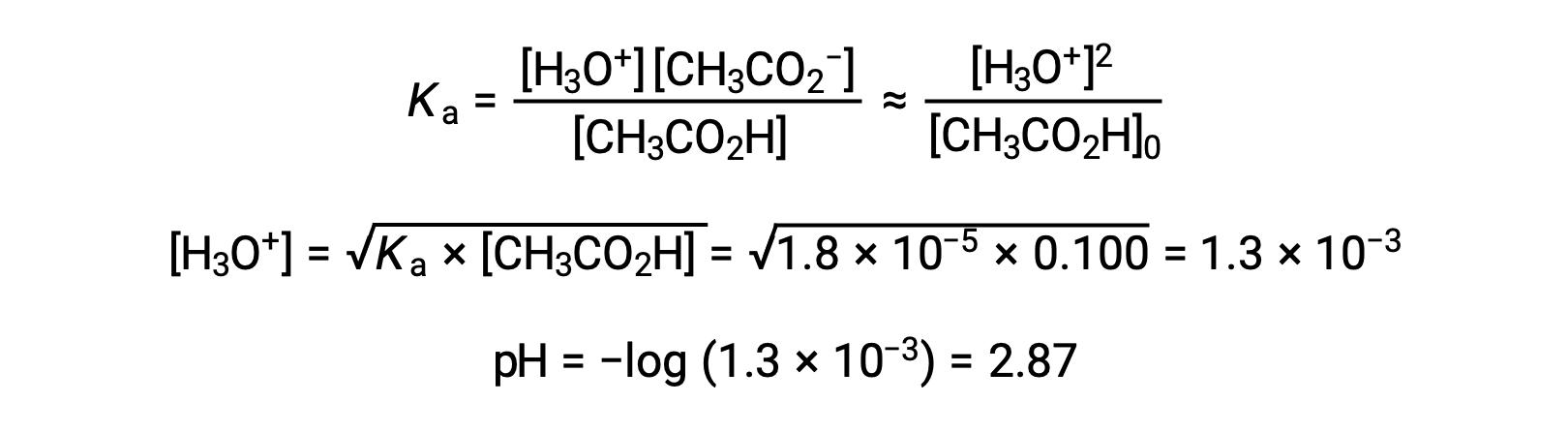
(b) The acid and titrant are both monoprotic and the sample and titrant solutions are equally concentrated; thus, this volume of titrant represents the equivalence point. Unlike the strong-acid example, the reaction mixture in this case contains a weak conjugate base (acetate ion). The solution pH is computed considering the base ionization of acetate, which is present at a concentration of
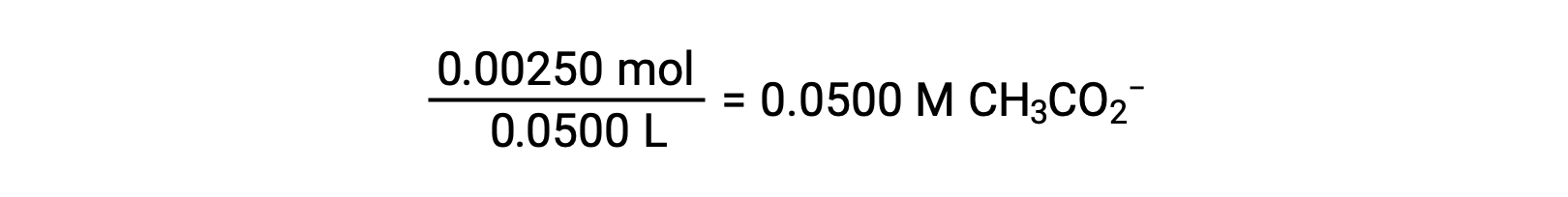
Base ionization of acetate is represented by the equation
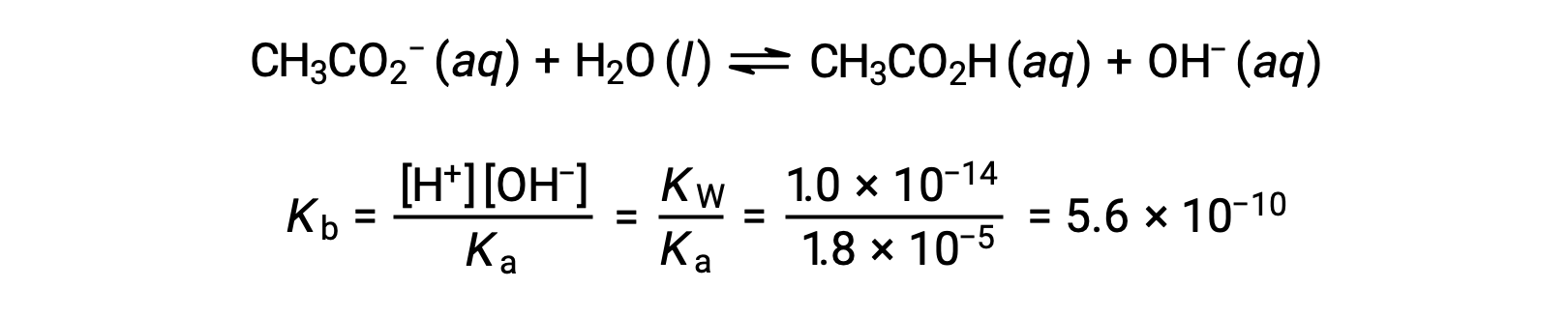
Assuming x << 0.0500, the pH may be calculated via the usual ICE approach:
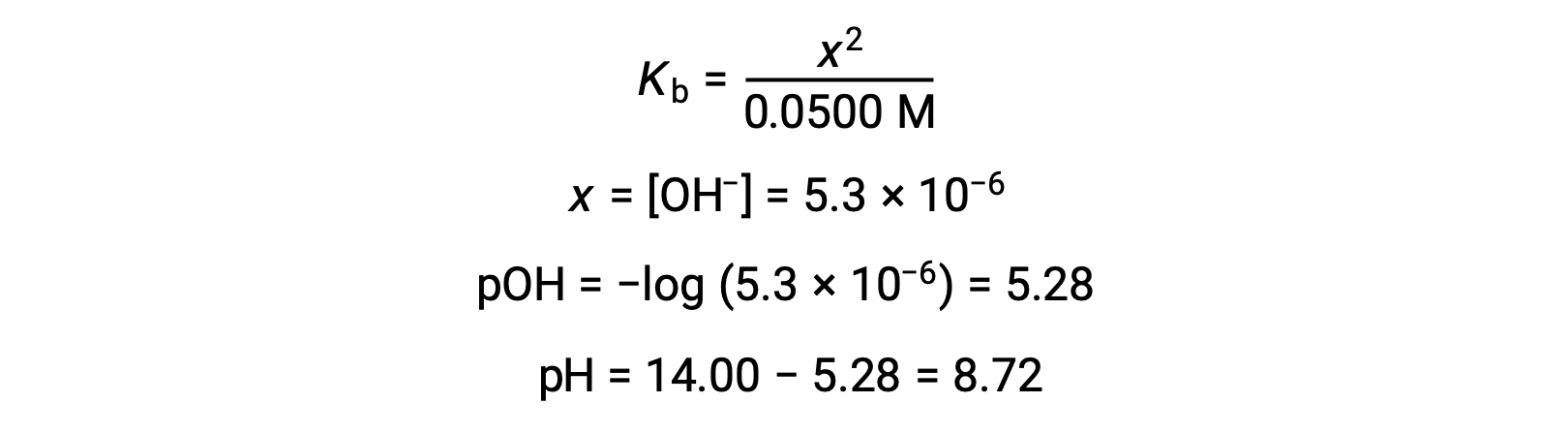
Note that the pH at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base.
(c) Titrant volume = 12.50 mL. This volume represents one-half of the stoichiometric amount of titrant, and so one-half of the acetic acid has been neutralized to yield an equivalent amount of acetate ion. The concentrations of these conjugate acid-base partners, therefore, are equal. A convenient approach to computing the pH is use of the Henderson-Hasselbalch equation:
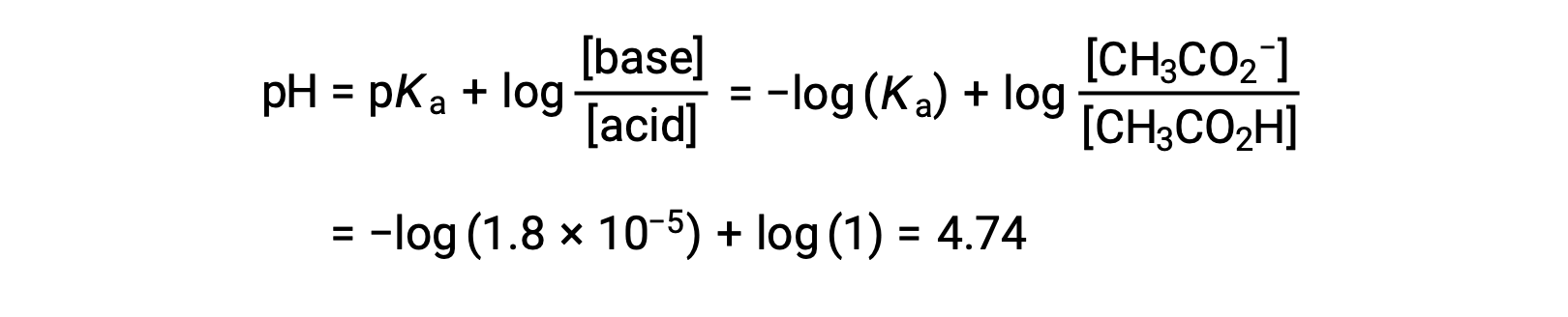
(pH = pKa at the half-equivalence point in a titration of a weak acid)
(d) Titrant volume = 37.50 mL. This volume represents a stoichiometric excess of titrant, and a reaction solution containing both the titration product, acetate ion, and the excess strong titrant. In such solutions, the solution pH is determined primarily by the amount of excess strong base:
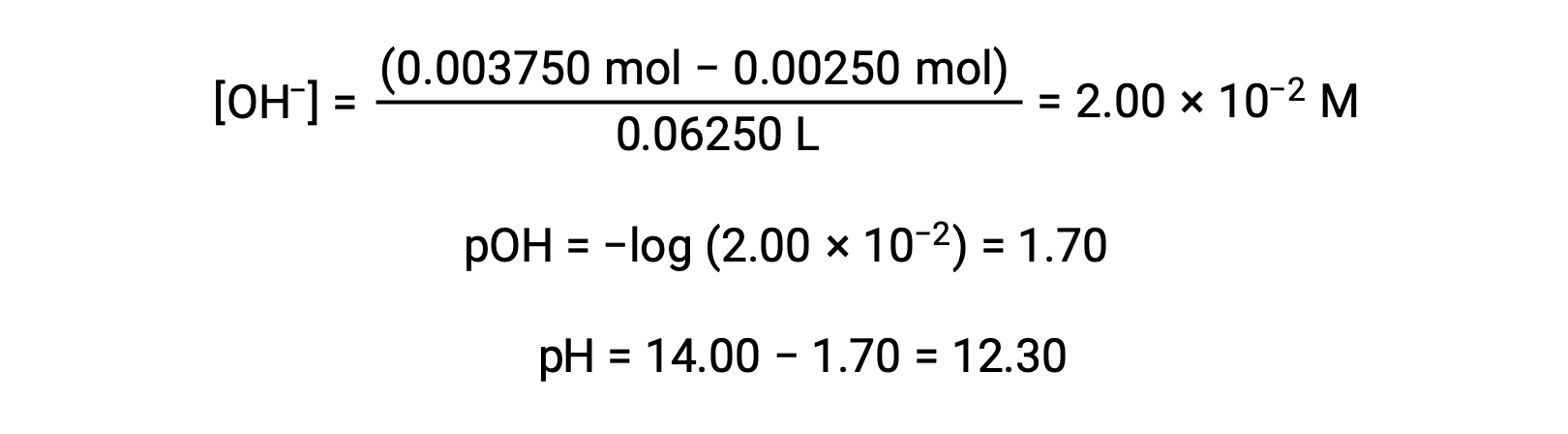
This text is adapted from Openstax, Chemistry 2e, Section 14.7: Acid-base Titrations.
From Chapter 16:

Now Playing
16.7 : Titration Calculations: Weak Acid - Strong Base
Acid-base and Solubility Equilibria
43.9K Views

16.1 : Common Ion Effect
Acid-base and Solubility Equilibria
41.1K Views

16.2 : Buffers
Acid-base and Solubility Equilibria
163.6K Views

16.3 : Henderson-Hasselbalch Equation
Acid-base and Solubility Equilibria
68.3K Views

16.4 : Calculating pH Changes in a Buffer Solution
Acid-base and Solubility Equilibria
52.7K Views

16.5 : Buffer Effectiveness
Acid-base and Solubility Equilibria
48.5K Views

16.6 : Titration Calculations: Strong Acid - Strong Base
Acid-base and Solubility Equilibria
29.1K Views

16.8 : Indicators
Acid-base and Solubility Equilibria
47.9K Views

16.9 : Titration of a Polyprotic Acid
Acid-base and Solubility Equilibria
95.8K Views

16.10 : Solubility Equilibria
Acid-base and Solubility Equilibria
52.1K Views

16.11 : Factors Affecting Solubility
Acid-base and Solubility Equilibria
33.1K Views

16.12 : Formation of Complex Ions
Acid-base and Solubility Equilibria
23.3K Views

16.13 : Precipitation of Ions
Acid-base and Solubility Equilibria
27.6K Views

16.14 : Qualitative Analysis
Acid-base and Solubility Equilibria
22.0K Views

16.15 : Acid-Base Titration Curves
Acid-base and Solubility Equilibria
126.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved