All living things are formed mostly of carbon compounds called organic compounds. The category of organic compounds includes both natural and synthetic compounds that contain carbon. Although a single, precise definition has yet to be identified by the chemistry community, most agree that a defining trait of organic molecules is the presence of carbon as the principal element, bonded to hydrogen and other carbon atoms. However, some carbon-containing compounds such as carbonates, cyanides, and simple oxides (CO and CO2) are not classified as organic compounds.
Organic compounds are key components of plastics, soaps, perfumes, sweeteners, fabrics, pharmaceuticals, and many other substances used daily. Organic compounds include compounds originating from living organisms and those synthesized by chemists. The existence of a wide array of organic molecules is a consequence of carbon atoms' ability to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
Hydrocarbons
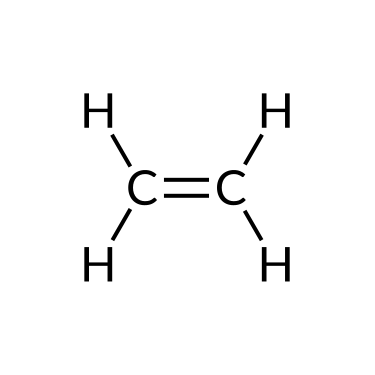
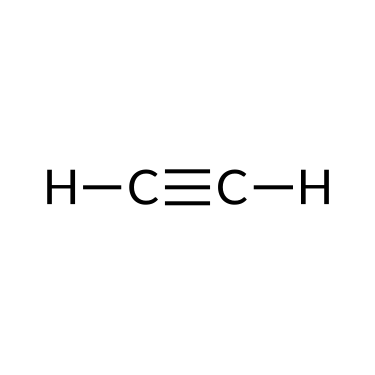
The simplest organic compounds contain only the elements carbon and hydrogen and are called hydrocarbons. Hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules. Those containing only single bonds are called alkanes, while those containing double or triple bonds are alkenes and alkynes, respectively. Although all hydrocarbons are composed of only two types of atoms (carbon and hydrogen), there is a wide variety of hydrocarbons because they may consist of varying lengths of chains, branched chains, and rings of carbon atoms, or combinations of these structures.
|
|
|
|
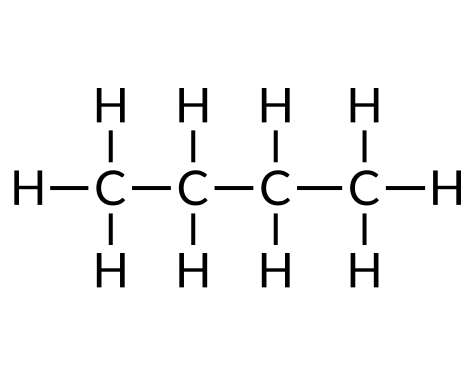
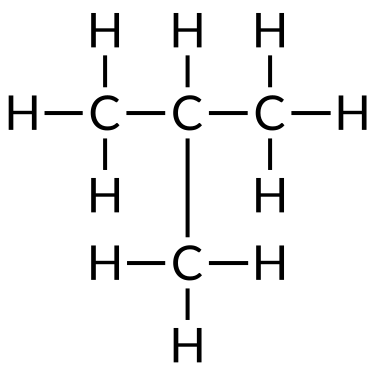
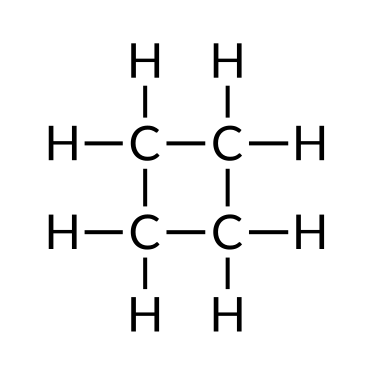
| Butane (C4H10) | Isobutane (C4H10) | Cyclobutane (C4H8) |
Hydrocarbons are used every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. Alkanes, or saturated hydrocarbons, contain only single covalent bonds between their carbon atoms. Properties such as melting point and the boiling point usually change predictably as the number of carbon and hydrogen atoms in the molecules change.
To name a simple alkane, first identify the base name depending on the number of carbon atoms in the chain (meth = 1, eth = 2, prop = 3, but = 4, pent = 5, hex = 6, hept = 7, oct = 8, non = 9, and dec = 10). The base name is followed with a suffix — determined by whether the hydrocarbon is an alkane (-ane ), alkene (-ene ), or alkyne (-yne ). For example, a two-carbon alkane is called ethane; a three-carbon alkane is called propane; and a four-carbon alkane is called butane. Longer chains are named as follows: pentane (5-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10).
Alkenes and alkynes are unsaturated hydrocarbons containing double bonds and triple bonds, respectively, between at least two carbon atoms. Their nomenclature follows the same steps as that of alkane: base name + suffix. For example, a two-carbon alkene chain is called ethene, and a two-carbon alkyne is called ethyne; a three-carbon alkene is called propene, and a three-carbon alkyne is called propyne, and so on.
|
|
|
|
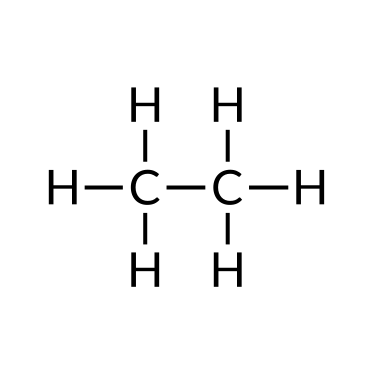
| Ethane (C2H6) | Ethene (C2H4) | Ethyne (C2H2) |
Functionalized Hydrocarbons
Incorporation of a functional group into carbon- and hydrogen-containing molecules leads to new families of compounds called functionalized hydrocarbons. The functional group is a characteristic atom or group of atoms that primarily determines the properties of hydrocarbon derivatives.
One type of functional group is the –OH group. Compounds that have an –OH functional group are alcohols. The name of the alcohol comes from the hydrocarbon from which it was derived. By convention, the hydrocarbon portion of the molecule is designated as 'R'; so the general formula of an alcohol is R–OH. The final ‘–e’ in the name of the hydrocarbon is replaced by ‘–ol’. In the case of a branched alcohol, the carbon atom to which the –OH group is bonded is indicated by a number placed before the name. Other common functional groups are listed below. A group of compounds containing the same functional group forms a family.
| Family | Functional group | Example | Formula | Name |
| Alcohols | C3H8O | Propanol | ||
| Ethers | C2H6O | Dimethyl ether | ||
| Aldehydes | C3H6O | Propanal | ||
| Ketones | C3H6O | Propanone (acetone) | ||
| Carboxylic acids | C3H6O2 | Propanoic acid | ||
| Esters | C4H8O2 | Ethyl acetate | ||
| Amines | C3H9N | Propylamine |
Ethers are compounds that contain the functional group –O–, with the general formula R–O–R’.
Another class of organic molecules contains a carbon atom connected to an oxygen atom by a double bond, commonly called a carbonyl group. The carbon in the carbonyl group can attach to two other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids, and esters).
Functional groups related to the carbonyl group include the –CHO group of an aldehyde, the –CO– group of a ketone, the –CO2H group of a carboxylic acid, and the –CO2R group of an ester. The carbonyl group, a carbon-oxygen double bond, is the key structure in these classes of organic molecules. Aldehydes contain at least one hydrogen atom attached to the carbonyl carbon atom, ketones contain two carbon groups attached to the carbonyl carbon atom, carboxylic acids contain a hydroxyl group attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom. All of these compounds contain oxidized carbon atoms relative to the carbon atom of an alcohol group.
The addition of nitrogen into an organic framework leads to two families of molecules, namely amines and amides. Compounds containing a nitrogen atom bonded in a hydrocarbon framework are classified as amines. Compounds that have a nitrogen atom bonded to one side of a carbonyl group are classified as amides. Amines are a basic functional group. Amines and carboxylic acids can combine in a condensation reaction to form amides.
This text is adapted from Openstax, Chemistry 2e, Section 20: Introduction, Openstax, Chemistry 2e, Section 20.1: Hydrocarbons, Openstax, Chemistry 2e, Section 20.2: Alcohols and Ethers, Openstax, Chemistry 2e, Section 20.2: Aldehydes, Ketones, Carboxylic Acids, and Esters, and Openstax, Chemistry 2e, Section 20.2: Amines and Amides.
From Chapter 3:

Now Playing
3.7 : Organic Compounds
Molecules, Compounds, and Chemical Equations
49.3K Views

3.1 : Molecules and Compounds
Molecules, Compounds, and Chemical Equations
49.1K Views

3.2 : Chemical Formulas
Molecules, Compounds, and Chemical Equations
46.7K Views

3.3 : Molecular Models
Molecules, Compounds, and Chemical Equations
36.1K Views

3.4 : Classification of Elements and Compounds
Molecules, Compounds, and Chemical Equations
61.7K Views

3.5 : Ionic Compounds: Formulas and Nomenclature
Molecules, Compounds, and Chemical Equations
63.8K Views

3.6 : Molecular Compounds: Formulas and Nomenclature
Molecules, Compounds, and Chemical Equations
41.1K Views

3.8 : Formula Mass and Mole Concepts of Compounds
Molecules, Compounds, and Chemical Equations
58.8K Views

3.9 : Experimental Determination of Chemical Formula
Molecules, Compounds, and Chemical Equations
35.5K Views

3.10 : Chemical Equations
Molecules, Compounds, and Chemical Equations
59.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved