13.12 : Enzymes
Inside living organisms, enzymes act as catalysts for many biochemical reactions involved in cellular metabolism. The role of enzymes is to reduce the activation energies of biochemical reactions by forming complexes with its substrates. The lowering of activation energies favor an increase in the rates of biochemical reactions.
Enzyme deficiencies can often translate into life-threatening diseases. For example, a genetic abnormality resulting in the deficiency of the enzyme G6PD (glucose-6-phosphate dehydrogenase) adversely affects the metabolic pathway supplying NADPH to cells.
A disruption in this metabolic pathway can reduce glutathione in red blood cells causing damage to other enzymes and proteins like hemoglobin. The excessive metabolization of hemoglobin builds up the bilirubin level, which leads to jaundice, a condition that can become severe. Hence, people who suffer from G6PD deficiency must avoid certain foods and medicines containing chemicals that could trigger damage to their glutathione-deficient red blood cells.
Enzyme Function and Structure
Enzymes are grouped into different classes based on the specific function they perform. For example, oxidoreductases are involved in redox reactions, while transferases catalyze the transfer of functional groups. Bond formation with ATP hydrolysis requires ligases, whereas hydrolysis reactions and double bonds formation are catalyzed by hydrolases and lyases, respectively. Isomerase enzymes usually catalyze isomerization reactions.
Enzymes generally possess active sites. These are specific regions on the molecule with a conformation that favors the enzyme to bind to a specific substrate (a reactant molecule) to form an enzyme-substrate complex or the reaction intermediate.
Two models—the lock-and-key model and the induced-fit model—attempt to explain the working of an active site (Figure 1). The most simplistic lock-and-key hypothesis suggests that the active site and the molecular shape of the substrate are complementary—fitting together like a key in a lock (Figure 1a). On the other hand, the induced-fit hypothesis suggests that the enzyme molecule is flexible and changes shape to accommodate a bond with the substrate (Figure 1b).
However, both the lock-and-key model and the induced-fit model account for the fact that enzymes can only bind with specific substrates and catalyzes only a particular reaction.
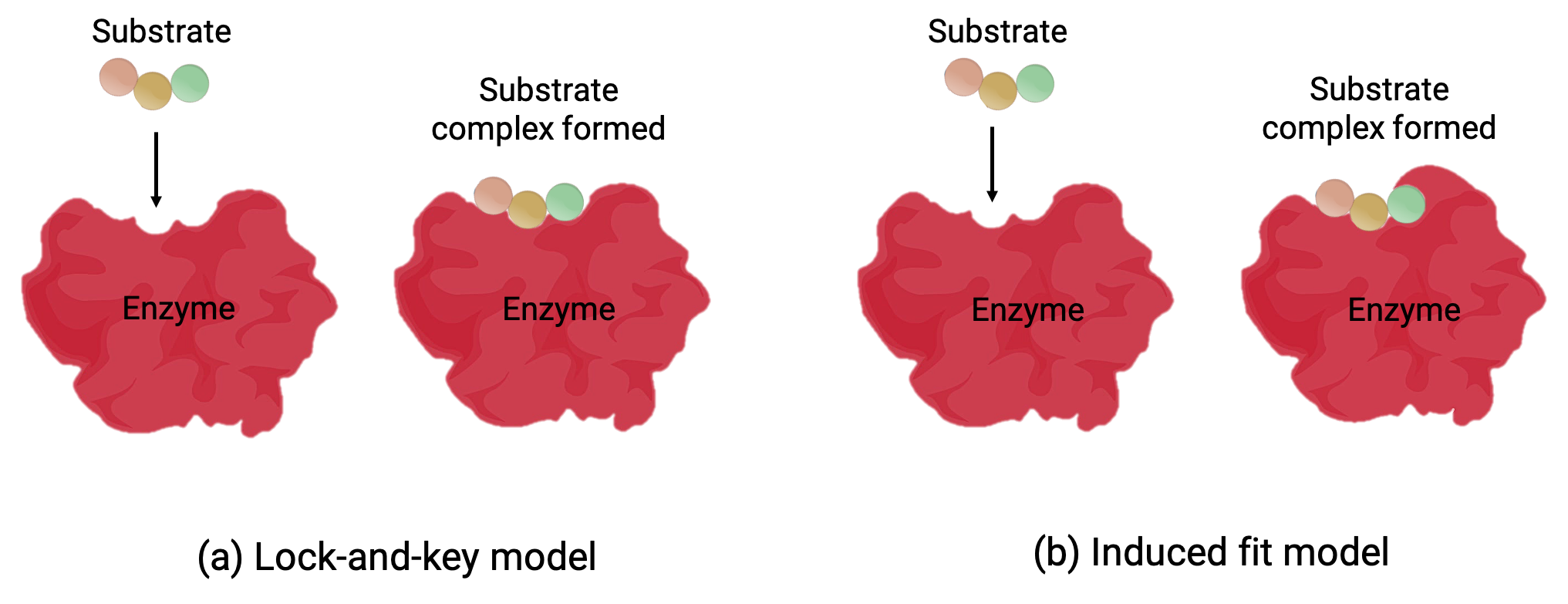
Figure 1 (a) According to the lock-and-key model, the shape of an enzyme’s active site is a perfect fit for the substrate. (b) According to the induced fit model, the active site is somewhat flexible, and can change shape in order to bond with the substrate.
Enzyme Inhibitors
The activity of enzymes can also be interrupted by the process of enzyme inhibition. There are several common types of enzyme inhibition.
During competitive inhibition, a molecule (natural or synthetic) other than the substrate directly binds to the enzyme's active site. The structural and chemical similarity of the inhibitor to the substrate facilitates its binding to the active site. Such competitive inhibitors, thus, compete with substrates, preventing them from binding to the enzyme. Most often, increasing substrate concentration can suppress the effects of competitive inhibition.
In non-competitive inhibition, a molecule (natural or synthetic) binds to an allosteric (other) region of the enzyme, different from its active site. The inhibitor-binding causes a conformational change to the enzyme's active site, resulting in a decrease in the enzyme's ability to catalyze the reaction. Unlike competitive inhibition, an increase in substrate concentration does not mitigate the inhibitory effects of non-competitive inhibition.
Part of this text is adapted from Openstax, Chemistry 2e, Section 12.7: Catalysis.
From Chapter 13:

Now Playing
13.12 : Enzymes
Chemical Kinetics
80.4K Views

13.1 : Reaction Rate
Chemical Kinetics
50.9K Views

13.2 : Measuring Reaction Rates
Chemical Kinetics
24.3K Views

13.3 : Concentration and Rate Law
Chemical Kinetics
29.8K Views

13.4 : Determining Order of Reaction
Chemical Kinetics
54.8K Views

13.5 : The Integrated Rate Law: The Dependence of Concentration on Time
Chemical Kinetics
34.2K Views

13.6 : Half-life of a Reaction
Chemical Kinetics
34.1K Views

13.7 : Temperature Dependence on Reaction Rate
Chemical Kinetics
81.0K Views

13.8 : Arrhenius Plots
Chemical Kinetics
38.3K Views

13.9 : Reaction Mechanisms
Chemical Kinetics
25.1K Views

13.10 : Rate-Determining Steps
Chemical Kinetics
31.5K Views

13.11 : Catalysis
Chemical Kinetics
26.5K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved