A subscription to JoVE is required to view this content. Sign in or start your free trial.
Misfolding-Prone Protein Degradation Assay: A Technique to Monitor Misfolded Protein Degradation Using Cycloheximide Treatment and Detergent Fractionation
In This Article
Overview
This video describes a degradation assay for misfolded proteins using cycloheximide treatment along with detergent fractionation. This method aids in studying the dynamics of misfolded proteins and uncovering the in-depth mechanisms of protein turnover.
Protocol
1. Preparation of Reagent
- Prepare cell lysis buffer (50 mM Tris, pH 8.8, 100 mM NaCl, 5 mM MgCl2, 0.5% NP-40). Supplement 2 mM DTT, 1x complete protease cocktail, and 250 IU/ml benzonase before use.
- Prepare pellet buffer (20 mM Tris, pH 8.0, 15 mM MgCl2). Supplement 2 mM DTT, 1x complete protease cocktail, and 250 IU/ml benzonase before use.
- Prepare 3x boiling buffer (6% SDS, 20 mM Tris, pH 8.0). Supplement 150 mM DTT before use.
- Prepare low-fluorescence DMEM medium for assays using a microplate fluorescence reader. Mix 25 mM glucose, 0.4 mM glycine, 0.4 mM arginine, 0.2 mM cysteine, 4.0 mM glutamine, 0.2 mM histidine, 0.8 mM isoleucine, 0.8 mM leucine, 0.8 mM lysine, 0.2 mM methionine, 0.4 mM phenylalanine, 0.4 mM serine, 0.8 mM threonine, 0.078 mM tryptophan, 0.4 mM tyrosine, 0.8 mM valine, 1.8 mM CaCl2, 0.81 mM MgSO4, 5.33 mM KCl, 44.0 mM NaHCO3, 110 mM NaCl, 0.9 mM NaH2PO4. Adjust the pH of the solution using HCl or NaOH to pH 7.4. Sterilize the medium through filtration.
NOTE: This medium contains components of the standard DMEM medium with high glucose except that Fe (NO3)3, vitamins, and Phenol Red are omitted. Phenol Red, riboflavin, and pyridoxal in regular DMEM culture medium significantly interfere with the detection of fluorescence signal. Culture medium without those components is crucial for successful live cell GFP imaging. The medium is stable for 12 months when stored refrigerated.
2. Degradation Assay of Atxn1 82Q GFP
- Plate approximately 3 x 105 HeLa cells into 35 mm plates with DMEM medium supplemented with 10% fetal bovine serum (FBS), so that after O/N culturing cells grow to a confluence of 40-60% at the time of transfection.
NOTE: The number of plates needed is based on the number of time points and treatments described below. - Transfect HeLa cells with 0.3 µg of Atxn1 82Q-GFP/pRK5 plasmid into each well using transfection reagent according to manufacturer's instruction. Make a master transfection mix containing DNA and transfection reagent and aliquot it for each well.
- 4-5 hr post-transfection, examine the live cells under an inverted fluorescent microscope for GFP expression with excitation wavelength 450-490 nm. Return cells back to incubator after imaging.
NOTE: Observe both diffused GFP signals and small speckles of GFP signals in nucleus at this time. - Remove the medium by vacuum aspiration and add 2 ml fresh DMEM containing 50 µg/ml Cycloheximide (CHX). Treat cells for 0, 4, 8, 12, and 16 hr with CHX before harvesting. To examine proteasomal degradation, include proteasome inhibitor MG132 (10 µM) in one plate treated for 16 hr as a control.
- Harvest one plate of cells at each time point. Remove the medium by vacuum aspiration and wash the plates twice with 3 ml ice-cold 1x phosphate-buffered saline (PBS). Snap-freeze the plate on dry ice.
- After the last time point (16 hr), scrape the frozen cells (from all time points) into 150 µl of ice-cold cell lysis buffer and incubated on ice for 30 min.
- Centrifuge the cell lysates in a benchtop centrifuge at 17,000 x g for 15 min at 4 °C.
- Transfer the supernatant, which contains NP-40-soluble (NS) proteins, to another tube using a pipette.
NOTE: Use supernatants for measuring protein concentrations by Bradford assay if needed. - Rinse the pellets by gently adding approximately 200 µl 1x PBS to the tubes without disturbing the pellets. Carefully remove PBS by vacuum aspiration or pipette. Re-suspend them in 150 µl ice-cold cell pellet buffer, followed by 15-30 min incubation on ice.
NOTE: The resuspended pellet fraction contains NP-40 insoluble (NI) proteins. See Figure 1B for a diagram of detergent fractionation. - Add 75 µl of 3x boiling buffer into NS fractions and NP-40 insoluble (NI) fractions resuspended from the pellets. Heat the samples at 95 °C on a heat block for 5 min.
NOTE: Clumps in the NP-40 insoluble fractions are dissolved after heating and samples will become clear. - Add SDS-gel loading buffer to an aliquot of boiled NS and NI. Load equal volume of samples collected from all time points to SDS-PAGE gel. The volume loaded corresponds to approximately 20 μg of NS from sample collected at Time 0. NOTE: NS, as well as SDS-soluble (SS) protein from NI faction, can be resolved by SDS separating gel. In contrast, SDS-resistant (SR) aggregates from NI fraction are stuck in the gel loading wells (Figure 1B). To improve western blot detection, double volume of NI can be loaded.
- Detect NS and SS Atxn1 82Q-GFP by western blot using anti-GFP antibody and enhanced chemiluminescence (ECL).
NOTE: Atxn1 82Q in the SS fraction is generally less compared to the NS fraction when using this protocol. Longer ECL exposure is needed for optimal signals. - Examine SR Atxn1 82Q from the pellet fraction by filter retardation assays using a dot-blot apparatus (Figure 1B). Briefly, set up a dot-blot apparatus holding a 0.2 µm cellulose acetate membrane. Load 80-120 µl of boiled NI into each well of the dot-blot apparatus.
NOTE: As only small amounts of SR aggregates are formed in cells, for the dot blot assay, load a volume of the SR fraction that is approximately 10-15 times of the volume of the NS fraction used for western blot analysis.- After filtering the samples through the membrane by vacuum, Atxn1 82Q-GFP aggregates stuck on the membrane can be detected by anti-GFP immunoblotting.
Results
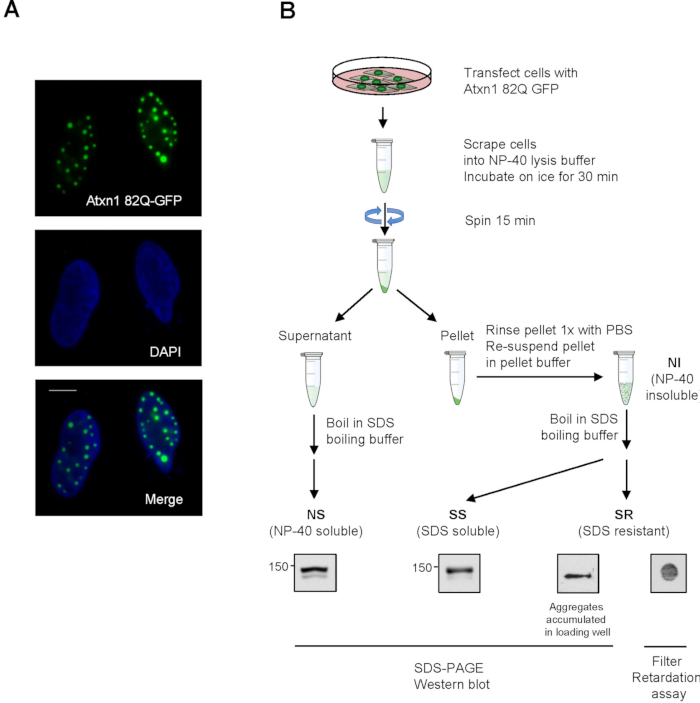
Figure 1. Detection of Atxn1 82Q-GFP by fluorescent microscopy and detergent fractionation. (A) HeLa cells were transfected with Atxn1 82Q-GFP and stained with DAPI. Individual and merged images are shown. Scale bar = 10 µm. (B) A diagram showing the detergent fractionation of Atxn1-82Q expressing cells as described in protocol 2. ...
Disclosures
Materials
| Name | Company | Catalog Number | Comments |
| Dulbecco's Modified Eagle Medium | Life Technologies | 11995-092 | |
| Fetal Bovine Serum | Life Technologies | 10082147 | |
| NP-40 | Sigma-Aldrich | NP40S-500ML | |
| SDS | Sigma-Aldrich | L3771 | |
| Cycloheximide | Sigma-Aldrich | C7698 | |
| Complete Protease Inhibitor Cocktail Tablets | Roche Boehringer Mannheim | 4693159001 | |
| Bio-Dot Apparatus | Bio-Rad | 1706545 | |
| Living Colors GFP Monoclonal Antibody | Clonetech | 632375 | |
| Cellulose acetate membrane 0.2 µm | Sterlitech CA | 23001 |
This article has been published
Video Coming Soon
Source: Guo, L. et al., Assays for the Degradation of Misfolded Proteins in Cells. J. Vis. Exp. (2016).
Copyright © 2025 MyJoVE Corporation. All rights reserved