A subscription to JoVE is required to view this content. Sign in or start your free trial.
Calcification Assay to Measure Calcium Concentration in Aortic Valve Interstitial Cells
In This Article
Overview
This video demonstrates an assay technique for assessing the calcification of the aortic valve interstitial cells in vitro. The assay helps to quantify the calcium concentration using the arsenazo III reagent, a calcium-sensitive dye that reacts with calcium ions to form a purple-colored calcium-arsenazo complex.
Protocol
All animal procedures described here have been approved by Icahn School of Medicine at Mount Sinai institutional core and use committee.
1. Preparation before valve cell isolation from adult mice
- Clean and sterilize all the surgical instruments shown in Figure 1A by using 70% v/v ethanol and subsequently autoclaving them for 30 min. clean the surgical workspace with 70% ethanol.
- Add 500 µL of penicillin-streptomycin to 50 mL of 10 mm HEPES. Prepare an aliquot of 50 mL of 1x phosphate buffered saline (PBS). Keep the solutions on ice.
- Prepare 1 mg/mL and 4.5 mg/mL collagenase solutions and use 5 mL of each solution in 15 mL tubes to perform the entire procedure. To prepare 5 mL of 1 mg/mL collagenase, mix 5 mg of collagenase with 2.5 mL of Dulbecco's Modified Eagle Medium (DMEM, fetal bovine serum (FBS)-free) and 2.5 mL of 10 mM HEPES supplemented with antibiotics (1% penicillin-streptomycin from step 1.2). Filter the solutions through a 0.22 µm filter to remove any contamination.
NOTE: Keep the solutions on ice to protect the enzymes. - Warm the DMEM solution to 37 °C before use in all the steps described below. Prepare complete medium by supplementing DMEM with 1% penicillin-streptomycin, 1% sodium pyruvate, 5 mL of 200 mM L-glutamine, 1 mL of mycoplasma elimination reagent (see the Table of Materials), and 10% FBS.
2. Isolation of valve cells
- To obtain 105 cells for the experiment, use five 8-week-old mice (minimum of three). Place the mouse in an induction chamber along with a small piece of tissue paper soaked with 1 mL of isoflurane, but do not allow contact with the tissue. To confirm that the animal is fully anesthetized; check for toe pinch reflex, and then euthanize the mouse by cervical dislocation. Use isoflurane to alleviate any pain prior to the cervical dislocation as the procedure described below is terminal.
- Place the mouse on a dissecting platform, and fix the paws with cannulas to hold it in place. Clean the chest and the abdomen with ethanol; open the abdomen and the chest with scissors. With small surgical scissors, cut between the left atrium and the left ventricle to exsanguinate the mouse. Perfuse the heart with 10 mL of cold 1x PBS to remove blood from the heart.
- Cut the heart, and keep 3 mm from the ascending aorta as shown in Figure 1B. Dissect the aortic valve under a stereomicroscope. Cut the heart horizontally in the middle of the ventricles (Figure 1C). Cut the left ventricle toward the aorta, and carefully dissect the aortic valve (Figure 1D-F). Pool the valves together in a small 35 mm tissue culture dish.
- Wash the isolated valves in a 75 mm cell culture dish with 5 mL of cold HEPES (10 mM) supplemented with antibiotics (1% penicillin-streptomycin) to remove blood (Figure 2). Prepare two 15 mL tubes of collagenase 1 mg/mL and 4.5 mg/mL as described above in step 1.3.
NOTE: After the dissection, manipulate the isolated valves in a sterile biosafety hood to minimize contamination. - Incubate the valves in collagenase type I (1 mg/mL) for 30 min at 37 °C with continuous shaking (Figure 2). Centrifuge the tube for 5 min at 150 × g, wash the pellet once with 2 mL of HEPES (10 mM), and vortex for 30 s at high speed. Pour the contents of this tube into a 35 mm culture dish, and carefully transfer the fragments of tissue using thin tweezers into a new tube.
NOTE: At this stage, the VICs are still not dissociated from the tissue, and the pellet contains pieces of tissue. To avoid contamination with endothelial cells, do not centrifuge after vortexing in step 2.5. - Incubate the pellet in a 15 mL tube with 5 mL of collagenase type I (4.5 mg/mL) at 37 °C under continuous agitation for 35 min. Re-suspend the cells with a 1 mL pipette to separate the cells, and centrifuge at 150 × g for 5 min at 4 °C.
- Discard the supernatant, and re-suspend the pellet in 2 mL of complete DMEM. Centrifuge at 150 × g for 5 min at 4 °C. Repeat this step twice to clean the cells.
NOTE: The pellet will still have some tissue fragments. - Re-suspend the pellet in 1 mL of complete medium, and plate the cells in one well of a 6-well cell culture dish in a minimum amount of medium to facilitate their attachment to the culture dish. Leave the cells, undisturbed, in a 37 °C incubator with 5% carbon dioxide.
- After 3 days, check the cells under the microscope to verify good growth close to the tissue debris. Once 1,000 cells are visible under the microscope, carefully remove the tissue debris with autoclaved tweezers, and change the medium.
NOTE: The plate should not be disturbed; if the required number of cells are not observed, place the cell culture dish back in the incubator for another 2 days. - When the cells are 70% confluent (2.5 × 105), trypsinize and then transfer them to a 75 mm tissue culture dish.
3. In vitro calcification assay
- Clean the hood with 70% ethanol, warm the DMEM medium to 37 °C.
- Seed 100,000 cells/condition into 6-well plates in complete DMEM, and culture for 24 h at 37 °C.
- Prepare the calcifying medium by mixing 2 mM of NaH2PO4, 10-7 M insulin, and 50 µg/mL ascorbic acid in DMEM with 5% FBS. For 93 mL of DMEM, add 5 mL of FBS, 1 mL of antibiotics (final concentration 1%), 1 mL of sodium pyruvate (100 mM), 27.5 mg of NaH2PO4, 5.8 µL of insulin, and 5 mg of ascorbic acid.
NOTE: Filter the solution using a 0.22 µm filter before use. - After 24 h, replace the supernatant medium with the calcifying medium. Incubate the cells for 7 days at 37 °C. On the 3rd day, replace with fresh calcifying medium, and place the plate back in the incubator to complete the 7 days of treatment.
- After 7 days, remove the medium, and wash the cells twice with 2 mL of 1x PBS. Incubate the cells in 1 mL of 0.6 N hydrochloric acid (HCl) for 24 h at 37 °C. Collect the HCl in a 1.5 mL tube, and evaporate it in a rotary evaporator. Re-suspend the contents of all the tubes in 60 µL of HCl.
NOTE: The drying procedure is important to concentrate the solution and to have the same volume for each condition. - Use a 96-well plate to measure calcium concentration by using Arsenazo III reagent, available in a ready-to-use kit (see the Table of Materials for more details).
- Prepare a calcium standard solution of 10 mg/dL concentration. Weigh 10 mg of calcium hydroxide (Ca(OH)2) and dissolve in 100 mL of distilled water.
- In a clear 96 well plate, pipet 2 µL of blank solution (HCl, 0.6 N), the standard solution, the sample per well (10 mg/dL), and the samples. Perform the experiment in triplicate to verify the pipetting variability. Add 200 µL of the reagent for each condition.
NOTE: Samples above 15 mg/dL should be diluted 1:1 with saline, re-assayed, and the result multiplied by two. - Incubate the reaction for 15 min at room temperature.
NOTE: The reaction is stable for 60 min. - Read and record the absorbance of the plate at 650 nm. Use the following formula to calculate the amount of calcium in the samples:
Calcium (mg/mL) = (Absorbance of sample/absorbance of standard) × Concentration of standard
Results
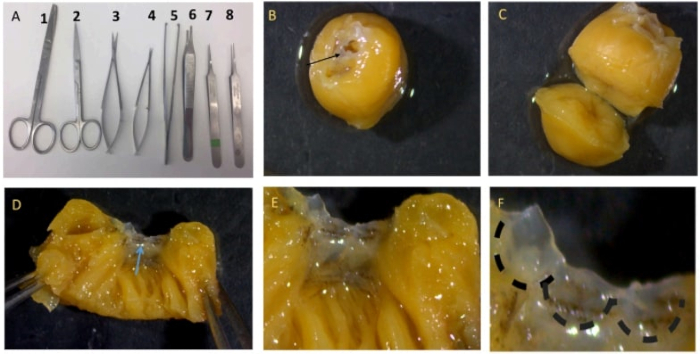
Figure 1: Description of valve dissection. (A) Representative image of all the surgical instruments needed for the dissection, scissors 2 is needed to open the skin of the mouse and scissors 3 to open the chest. Tweezers 5 and 6 are needed to hold the skin and open the chest. (B) Leave 3 mm of tissue from the aorta (black arrow). (C) Cut the hear...
Disclosures
Materials
| Name | Company | Catalog Number | Comments |
| 3 mm cutting edge scissors | F.S.T | 15000-00 | |
| Arsenazo-III reagent set | POINT SCIENTIFIC | C7529-500 | A kit to measure the concentration of calcium |
| Bonn Scissors | F.S.T | 14184-09 | |
| Calcium hydroxide | SIGMA -Aldrich | 31219 | |
| Collagenase type I (125 units/mg) | Thermofisher Scientific | 17018029 | |
| DMEM | Tthermofisher | 11965092 | |
| Extra fine graefe forceps | F.S.T | 11150-10 | |
| FBS | Gibco | 16000044 | |
| Fine forceps | F.S.T Dumont | ||
| HCl | SIGMA-ALDRICH | H1758 | |
| HEPES 1 M solution | STEMCELLS TECHNOLOGIES | ||
| L-Glutamine 100x | Thermofisher Scientific | A2916801 | |
| PBS 10x | SIGMA-ALDRICH | ||
| Penicillin streptomycin 100x | Thermofisher Scientific | 10378016 | |
| Sodium Pyruvate 100 mM | Thermofisher Scientific | 11360070 | |
| Standard pattern forceps | F.S.T | 11000-12 | |
| Surgical Scissors - Sharp-Blunt | F.S.T | 14008-14 | |
| Trypsin 0.05% | Thermofisher Scientific | 25300054 |
This article has been published
Video Coming Soon
Source: Bouchareb, R. et al., Isolation of Mouse Interstitial Valve Cells to Study the Calcification of the Aortic Valve In Vitro. J. Vis. Exp. (2021).
Copyright © 2025 MyJoVE Corporation. All rights reserved