Method Article
Optimized PCR-based Detection of Mycoplasma
In This Article
Summary
The LookOut Mycoplasma PCR Detection Kit utilizes the polymerase chain reaction (PCR), which is established as the method of choice for highest sensitivity in the detection of Mycoplasma, Acholeplasma, and Ureaplasma contamination in cell cultures and other cell culture derived biologicals.
Abstract
The maintenance of contamination-free cell lines is essential to cell-based research. Among the biggest contaminant concerns are mycoplasma contamination. Although mycoplasma do not usually kill contaminated cells, they are difficult to detect and can cause a variety of effects on cultured cells, including altered metabolism, slowed proliferation and chromosomal aberrations. In short, mycoplasma contamination compromises the value of those cell lines in providing accurate data for life science research.
The sources of mycoplasma contamination in the laboratory are very challenging to completely control. As certain mycoplasma species are found on human skin, they can be introduced through poor aseptic technique. Additionally, they can come from contaminated supplements such as fetal bovine serum, and most importantly from other contaminated cell cultures. Once mycoplasma contaminates a culture, it can quickly spread to contaminate other areas of the lab. Strict adherence to good laboratory practices such as good aseptic technique are key, and routine testing for mycoplasma is highly recommended for successful control of mycoplasma contamination.
PCR-based detection of mycoplasma has become a very popular method for routine cell line maintenance. PCR-based detection methods are highly sensitive and can provide rapid results, which allows researchers to respond quickly to isolate and eliminate contamination once it is detected in comparison to the time required using microbiological techniques. The LookOut Mycoplasma PCR Detection Kit is highly sensitive, with a detection limit of only 2 genomes per μl. Taking advantage of the highly specific JumpStart Taq DNA Polymerase and a proprietary primer design, false positives are greatly reduced. The convenient 8-tube format, strips pre-coated with dNTPs, and associated primers helps increase the throughput to meet the needs of customers with larger collections of cell lines.
Given the extreme sensitivity of the kit, great care must be taken to prevent inadvertent contamination of samples and reagents. The step-by-step protocol we demonstrate highlights the precautions and practices required for reliable mycoplasma detection. We also show and discuss typical results and their interpretation. Our goal is to ensure the success of researchers using the LookOut Mycoplasma PCR Detection Kit.
Protocol
1. Mycoplasma Detection
- Cell culture supernatants can be tested directly or the sample can prepared for use at a later date. To prepare for later use place 100 μl of supernatant in a sterile amplification tube and incubate at 95° C for 5 minutes. Once this is complete the sample can be stored at 2-8° C for up to one week. Just prior to running the sample briefly centrifuge (5 seconds) to pellet any cellular debris.
- To prepare the samples for PCR, determine the total volume of Jumpstart Taq DNA polymerase/rehydration buffer required for the reactions. We will be preparing 5 total reactions. Five reactions will require 2.5μl of Taq and 114.5μl of rehydration buffer. This will contain a minimum of one unit of Taq per reaction and 22.5μl of rehydration buffer per sample reaction and negative control plus 24.5μl of rehydration buffer for the positive control. 1 unit of DNA polymerase per reaction should be added to the appropriate volume of rehydration buffer. This will vary with the Taq used.
- Place the calculated volume of Taq DNA polymerase into a clean microcentrifuge tube and follow with the calculated volume of rehydration buffer. The DNA polymerase/rehydration buffer should be mixed gently by flicking the tube. This mixture should not be vortexed.
- To prepare the negative control and samples use the transparent reaction tubes provided in the kit. The reaction tubes provided in the kit already contain the nuclueotides, primers and internal control DNA. 23 μl of Jumpstart Taq DNA Polymerase/Rehydration buffer mix, as prepared in the previous steps, should be placed in each of the negative control and sample tubes. Add 2 μl of DNA free water to the negative control and add 2 μl of the sample to each of the sample tubes and label. Mix the contents by flicking the tubes. Contents should not be vortexed.
- To prepare the positive control use the pink reaction tubes provided in the kit. The reaction tubes provided in the kit already contain the nuclueotides, primers and internal control DNA. Add 25 μl of Jumpstart Taq DNA polymerase/Rehydration buffer mix, as prepared in the previous steps, to the reaction tubes and label. Mix the contents by flicking the tubes. Contents should not be vortexed. Incubate the negative control, positive control and sample tubes at room temperature for 5 minutes.
- For the PCR to take place the samples need to be placed in a thermal cycler. When using JumpStart Taq an activation step is not required. Place the reaction tubes in the thermal cycler. The cycles should be set as follows: 94°C for 30 seconds, 55°C for 30 seconds and 72°C for 40 seconds. These cycles will be run 40 times.
- Once the PCR cycles have completed the samples should be cooled to 4-8°C by removing them from the thermal cycler and placing them in ice. When the samples have cooled they should be loaded onto the agarose gel for electrophoresis. Loading gel and dye are not necessary as they are already present in the reaction tubes. Directly load 8 μl for each PCR into a separate lane. Stop the electrophoresis after migration of 2.5 - 3.0 cm.
2. Representative Results
The negative control should show a band at approximately 481 bp. This can be seen in the negative control column on the electrophoresis gel that is shown at the beginning of the video. The absence of the negative control band at 481 bp is a good indicator that the activity of the polymerase was not sufficienttaq used was not sensitive enough.
Mycoplasma positive samples will show bands in the range of 270 ± 8 bp. The band will be heavy for highly contaminated samples and faint for lightly contaminated samples. The variations in intensity can be seen in the positive sample columns on the electrophoresis gel that is shown at the beginning of the video. All samples contain an internal negative control to demonstrate that the PCR occurred as expected. It is normal to see the absence of the internal control on highly contaminated samples.
If there is not a band in the positive range or a band in the negative range on your sample this is a good indicator that your samples are inhibited. If the sample is inhibited the PCR inhibitors can be removed by performing a DNA extraction.
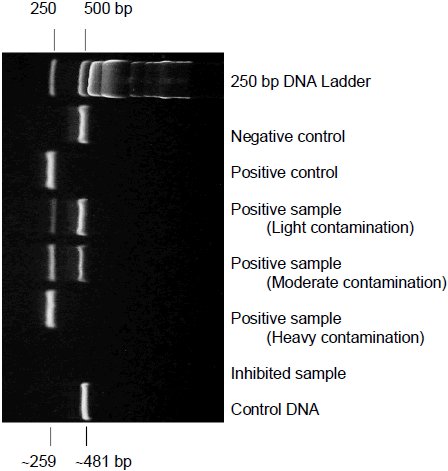
Figure 1. Relevant Amplicon bands: Results of detecting mycoplasma by PCR are shown. Lane 2 is the negative control, which should show a band at approximately 481 bp. The absence of the negative control band at 481 bp is a good indicator that the taq used was not sensitive enough. Mycoplasma-positive samples will show bands in the range of 270 + 8 bp as shown in lanes 3 - 6. The band will be heavy for highly contaminated samples (lane 6) and faint for lightly contaminated samples (lane 4). All samples contain an internal negative control (481 bp) to demonstrate that the PCR occurred as expected. It is normal to see the absence of the internal control on highly contaminated samples.
Discussion
Because of the extreme sensitivity of the kit, when performed correctly the kit should yield precise results indicating whether or not your sample has mycoplasma contamination. The kit has been optimized for use with JumpStart Taq DNA Polymerase. Other taqs may be used when preparing the samples but the internal control band at 481 bp should be present to indicate proper sensitivity of the alternative taq. It is possible for the positive bands to exhibit various densities depending on the level of contamination. It is possible to observe the absence of the internal control band on heavily contaminated samples. Using caution and proper technique when preparing the samples to prevent contamination are crucial for proper. detection when using the LookOut Mycoplasma PCR Detection Kit.
Disclosures
The authors are employees of Sigma-Aldrich that produced reagents and tools used in this article.
Acknowledgements
Funded by Sigma-Aldrich
Materials
| Name | Company | Catalog Number | Comments |
| LookOut Mycoplasma PCR Detection Kit | Sigma-Aldrich | MP0035 | |
| JumpStart Taq DNA Polymerase | Sigma-Aldrich | D9307 | |
| GenElute Blood Genomic DNA kit | Sigma-Aldrich | MP0030 | |
| Nunc amplification tubes | Sigma-Aldrich | T0447 |
References
- Wirth, M., Hauser, H., Drexler, H. G. Specificity and Sensitivity of Polymerase Chain Reaction (PCR) in Comparison with Other Methods for the Detection of Mycoplasma Contamination in Cell Lines. Journal of Immunological Methods. 164, 91-100 (1993).
- Kong, H., Volokhov, D. V., George, J., Ikonomi, P., Chandler, D., Anderson, C., Chizhikov, V. Amplification of Cell Culture Enrichment for Improving the Sensitivity of Mycoplasma Detection Methods Based on Nucleic Acid Amplification Technology (NAT). Applied Microbiology and Biotechnology. 77, 223-232 (2007).
- Volokhov, D. V., Graham, L. J., Borson, K. A., Chizhikov, V. Mycoplasma Testing of Cell Substrates and Biologics: Review of Alternative Non-Microbiological Techniques. Molecular and Cellular Probes. , (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved