A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Rat Model of Blood-brain Barrier Disruption to Allow Targeted Neurovascular Therapeutics
In This Article
Summary
Blood-brain barrier disruption aids the delivery of certain drugs to the brain. Mannitol delivered intra-arterially shrinks cells surrounding blood vessels in order to physically disrupt the barrier.
Abstract
Endothelial cells with tight junctions along with the basement membrane and astrocyte end feet surround cerebral blood vessels to form the blood-brain barrier1. The barrier selectively excludes molecules from crossing between the blood and the brain based upon their size and charge. This function can impede the delivery of therapeutics for neurological disorders. A number of chemotherapeutic drugs, for example, will not effectively cross the blood-brain barrier to reach tumor cells2. Thus, improving the delivery of drugs across the blood-brain barrier is an area of interest.
The most prevalent methods for enhancing the delivery of drugs to the brain are direct cerebral infusion and blood-brain barrier disruption3. Direct intracerebral infusion guarantees that therapies reach the brain; however, this method has a limited ability to disperse the drug4. Blood-brain barrier disruption (BBBD) allows drugs to flow directly from the circulatory system into the brain and thus more effectively reach dispersed tumor cells. Three methods of barrier disruption include osmotic barrier disruption, pharmacological barrier disruption, and focused ultrasound with microbubbles. Osmotic disruption, pioneered by Neuwelt, uses a hypertonic solution of 25% mannitol that dehydrates the cells of the blood-brain barrier causing them to shrink and disrupt their tight junctions. Barrier disruption can also be accomplished pharmacologically with vasoactive compounds such as histamine5 and bradykinin6. This method, however, is selective primarily for the brain-tumor barrier7. Additionally, RMP-7, an analog of the peptide bradykinin, was found to be inferior when compared head-to-head with osmotic BBBD with 25% mannitol8. Another method, focused ultrasound (FUS) in conjunction with microbubble ultrasound contrast agents, has also been shown to reversibly open the blood-brain barrier9. In comparison to FUS, though, 25% mannitol has a longer history of safety in human patients that makes it a proven tool for translational research10-12.
In order to accomplish BBBD, mannitol must be delivered at a high rate directly into the brain's arterial circulation. In humans, an endovascular catheter is guided to the brain where rapid, direct flow can be accomplished. This protocol models human BBBD as closely as possible. Following a cut-down to the bifurcation of the common carotid artery, a catheter is inserted retrograde into the ECA and used to deliver mannitol directly into the internal carotid artery (ICA) circulation. Propofol and N2O anesthesia are used for their ability to maximize the effectiveness of barrier disruption13. If executed properly, this procedure has the ability to safely, effectively, and reversibly open the blood-brain barrier and improve the delivery of drugs that do not ordinarily reach the brain 8,13,14.
Protocol
1. Prepare Animal and Equipment for Procedure
- Before beginning surgery, prepare the surgical area and the animal. Make the carotid catheter by inserting a 23-gauge blunt needle into one end of 12" of PE50 tubing. Cut an approximately 45° bevel in the opposite end of the catheter. Sterilize equipment prior to the procedure. Wear a hair bonnet, surgical mask, and sterile gloves.
- Place a heating pad on the surface where the surgery will be performed. Activate it and allow it to warm. Cover the area with an absorbent bench pad.
- Verify that you have 25% mannitol in solution without crystals. If crystals are present, dissolve them by placing the vial in a hot water bath at 80 °C and agitating periodically.
- Record the mass of the animal.
- Make a propofol dilution for delivery at 0.8 mg/kg/min at a rate of 0.1 ml/min.
- Place the rat in the induction box for 3 min. Settings: 5% isoflurane, 2 L/min flow rate on ventilator.
- On top of a disposable towel, shave the neck and both inner thighs.
2. Intubate the Rat
- Place the animal back in the induction box for 3 min.
- Prepare your endotracheal tube with an IV catheter. For this size and strain of rat, we use an 18 ga catheter. The tube must be large enough to allow adequate airflow, but small enough to fit into the trachea without causing damage. Pull the needle out of the plastic catheter and carefully break the needle approx. 1 cm from its sharp tip. The bunted needle will be your stylus. Put the needle back into the catheter.
- Hang the rat by its teeth on an angled surface.
- Shine a light directly against the animal's neck and lined up between its ears.
- Insert a small spatula approx. 3 cm into the oropharynx. Lift upward while gently pulling the tongue out of the mouth.
- Visualize the opening of the trachea. You will see a small hole open and close with each breath.
- Gently insert catheter into the hole. You can sometimes feel the plastic enter the cartilage of the trachea. The trachea is sensitive. Do not force the catheter.
- Set the ventilator to 1 L/min and 60 bpm and the isoflurane to 2%.
- Connect the catheter to the ventilator and tape the tube to the operating surface.
- Verify the animal is anesthetized by performing a toe pinch.
3. Establish Propofol IV Anesthesia
- Secure the rat by taping all four limbs and the tail to the operating surface.
- Scrub the shaved neck and groin sites with chlorhexidine soap three times. Then scrub with chlorhexidine rinse three times.
- Attach a syringe of propofol to an IV extension set. Mount the syringe on an infusion pump.
- Using a scalpel, cut through the skin of the shaved thigh. Avoid cutting muscle.
- Open the incision with forceps to visualize the femoral vein.
- Pull away tissue over the artery to expose the vessel.
- Insert a 26 ga monoject veterinary IV catheter into vein. When the needle punctures the wall of the vein you will feel it become easier to push. Retract the needle and push the catheter into the vessel.
- Start the propofol infusion at 0.1 ml/min.
- 3-5 min after beginning the propofol infusion, switch the anesthesia gas to a mixture of 50% O2 and 50% N2O and shut off the isoflurane.
4. Expose the Bifurcation of the Common Carotid Artery
- Make a midline incision at the neck with scalpel blade.
- Cut between the two large salivary glands with scissors or scalpel.
- Dissect away tissue lateral to the midline to expose the carotid artery. Use forceps to open layers of tissue. You can use scissors to remove pieces that you expose with the forceps and determine to be minimally vascular. Control bleeding with gauze, cotton swabs, and cautery if necessary.
- Inject 3 ml saline IP if there has been moderate to heavy bleeding.
- Use retractors to pull tissue away from the carotid. Find the bifurcation.
- Expose the occipital artery just distal to the bifurcation. Cauterize it between forceps and separate it with an electrocautery.
- Use forceps to fully expose the carotid bifurcation and as much of the ECA as possible.
- Expose the superior thyroid artery branching off the ECA distal to the occipital artery. Cauterize it between forceps and separate it with an electrocautery.
5. Catheterize the External Carotid Artery
- Place 2 lengths of 4/0 silk under the ECA.
- Grab 1 length of suture with your hemostat and retract the ECA back caudally (toward the rat's feet).
- Take the other suture and tie as far cranially (toward the rat's mouth) as possible with a surgeon's knot. This forms a permanent ligature to sacrifice the ECA.
- Remove the first hemostat (the one that was applying caudal tension) and grab the other suture (the one that is knotted around the ECA) and apply gentle tension cranially with the hemostat.
- Tie another surgeon's knot loosely (do not secure - leave barely tied) distal to the bifurcation and proximal to the permanent ligature.
- Irrigate with 1% lidocaine to relax the artery.
- Fill your catheter with heparinized saline.
- Place a temporary vessel clip as close as possible to the bifurcation. Place vessel clip at an angle to maximize space for catheterization. Slide the loose knot toward the clip.
- Make a small arteriotomy (cut) in ECA just proximal to the ligature using microscissors. There should be no bleeding if the clip and ligature are secure.
- Using forceps to control the ECA walls, feed the beveled end of the catheter into the vessel.
- Secure the catheter with the loose suture knot. Tie the artery around the catheter.
- Remove the clip and clean the area with a cotton swab.
- Advance the catheter to about 1 mm distal to bifurcation. Hold the artery with forceps proximal to the knot to ensure the vessel is immobilized.
6. Administer Mannitol
- Fill an 8" IV extension set and a 6 ml syringe with mannitol without creating any air bubbles. Store this in the incubator at 37 °C. Note: work quickly with mannitol - it will readily precipitate out of solution at room temperature.
- In order to visualize BBBD, you may administer Evans blue dye and watch it enter the areas of the brain where the barrier has been disrupted. To do this, give the animal 2 ml/kg of 2% Evans Blue through a new IV site on leg contralateral to the propofol infusion.
- Attach the extension set to a 5 μm filter and fill it with mannitol to remove air bubbles. Then, attach this to the stopcock without creating bubbles.
- Administer mannitol at 0.09 ml/sec for 25 sec. Watch for the mannitol to reach equilibrium with the flow in common carotid at a point just proximal to the ICA. It should appear to pulse with the rat's heart rate. If the mannitol simply flows down toward the heart you have overcome the rat's blood pressure. This can cause life-threatening complications and poor BBBD.
Results
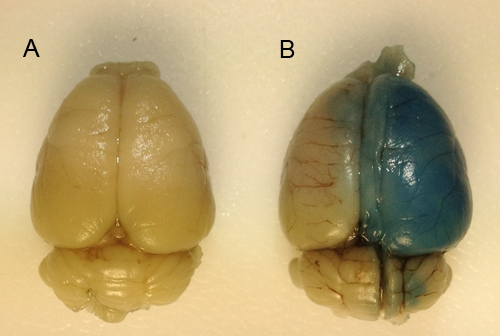
Figure 1. Visualizing blood-brain barrier disruption via Evans blue dye extravasation. Evans blue dye is a pigment that binds to albumin and is not extravasated into the brain under physiological conditions. Disruption of the blood-brain barrier on one side of the brain allows Evans blue to enter and stain the disrupted hemisphere blue while the non-disrupted hemisphere remains unchanged. Thus...
Discussion
There are a few means of maximizing the efficacy of BBBD. It is important to minimize bleeding during the cut-down phase. Blood pressure and heart rate can be affected by substantial bleeding and these factors are known to affect the degree of BBBD13. Bleeding can be reduced by using sutures to ligate major vessels, such as the superior thyroid and occipital arteries, which must be divided. Additionally, electrocautery can be used to divide vessels and dissect areas that have a rich blood supply. It is also im...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by the J.B. Marshall Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| Long Evans rat | Harlan Laboratories | 210-250 g, male | |
| PE 50 Tubing | Beckton-Dickinson | ||
| 18 gauge x 2.5" IV catheter | Terumo | For ET tube | |
| 30" IV extension sets | Abbott | ||
| 26 gauge veterinary IV catheter | Monoject | ||
| Evans blue dye | Sigma | E2129 | |
| Bipolar | Codman | ||
| Filter, 5 μm | Braun |
References
- Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R., Begley, D. J. Structure and function of the blood-brain barrier. Neurobiology of disease. 37, 13-25 (2010).
- Smith, Q. R. The Blood-Brain Barrier. 89, 193-208 (2003).
- Kroll, R. A., Neuwelt, E. A. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 42, 1083 (1998).
- Kroll, R. A., Pagel, M. A., Muldoon, L. L., Roman-Goldstein, S., Neuwelt, E. A. Increasing volume of distribution to the brain with interstitial infusion: dose, rather than convection, might be the most important factor. Neurosurgery. 38, 746-754 (1996).
- Butt, A. M., Jones, H. C. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain research. 569, 100-105 (1992).
- Inamura, T., Black, K. L. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. Journal of Cerebral Blood Flow & Metabolism. 14, 862-870 (1994).
- Matsukado, K., et al. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery. 39, 125 (1996).
- Kroll, R. A., et al. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery. 43, 879 (1998).
- Kinoshita, M., McDannold, N., Jolesz, F. A., Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proceedings of the National Academy of Sciences. 103, 11719-11723 (2006).
- Neuwelt, E. A., Dahlborg, S. A. Chemotherapy administered in conjunction with osmotic blood-brain barrier modification in patients with brain metastases. Journal of neuro-oncology. 4, 195-207 (1987).
- Doolittle, N. D., et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 88, 637-647 (2000).
- Guillaume, D. J., et al. Intra-arterial chemotherapy with osmotic blood-brain barrier disruption for aggressive oligodendroglial tumors: results of a phase I study. Neurosurgery. 66, 48 (2010).
- Remsen, L. G., et al. The influence of anesthetic choice, PaCO2, and other factors on osmotic blood-brain barrier disruption in rats with brain tumor xenografts. Anesthesia & Analgesia. 88, 559-559 (1999).
- Nilaver, G., et al. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proceedings of the National Academy of Sciences. 92, 9829 (1995).
- Fortin, D., McCormick, C. I., Remsen, L. G., Nixon, R., Neuwelt, E. A. Unexpected neurotoxicity of etoposide phosphate administered in combination with other chemotherapeutic agents after blood-brain barrier modification to enhance delivery, using propofol for general anesthesia, in a rat model. Neurosurgery. 47, 199 (2000).
- Neuwelt, E. A., et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. Journal of clinical oncology. 9, 1580 (1991).
- Remsen, L. G., Trail, P. A., Hellström, I., Hellström, K. E., Neuwelt, E. A. Enhanced delivery improves the efficacy of a tumor-specific doxorubicin immunoconjugate in a human brain tumor xenograft model. Neurosurgery. 46, 704 (2000).
- Neuwelt, E. A., Pagel, M. A., Kraemer, D. F., Peterson, D. R., Muldoon, L. L. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. Journal of Pharmacology and Experimental Therapeutics. 309, 594-599 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved