A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Protein WISDOM: A Workbench for In silico De novo Design of BioMolecules
In This Article
Summary
We developed computational de novo protein design methods capable of tackling several important areas of protein design. To disseminate these methods we present Protein WISDOM, an online tool for protein design (http://www.proteinwisdom.org). Starting from a structural template, design of monomeric proteins for increased stability and complexes for increased binding affinity can be performed.
Abstract
The aim of de novo protein design is to find the amino acid sequences that will fold into a desired 3-dimensional structure with improvements in specific properties, such as binding affinity, agonist or antagonist behavior, or stability, relative to the native sequence. Protein design lies at the center of current advances drug design and discovery. Not only does protein design provide predictions for potentially useful drug targets, but it also enhances our understanding of the protein folding process and protein-protein interactions. Experimental methods such as directed evolution have shown success in protein design. However, such methods are restricted by the limited sequence space that can be searched tractably. In contrast, computational design strategies allow for the screening of a much larger set of sequences covering a wide variety of properties and functionality. We have developed a range of computational de novo protein design methods capable of tackling several important areas of protein design. These include the design of monomeric proteins for increased stability and complexes for increased binding affinity.
To disseminate these methods for broader use we present Protein WISDOM (http://www.proteinwisdom.org), a tool that provides automated methods for a variety of protein design problems. Structural templates are submitted to initialize the design process. The first stage of design is an optimization sequence selection stage that aims at improving stability through minimization of potential energy in the sequence space. Selected sequences are then run through a fold specificity stage and a binding affinity stage. A rank-ordered list of the sequences for each step of the process, along with relevant designed structures, provides the user with a comprehensive quantitative assessment of the design. Here we provide the details of each design method, as well as several notable experimental successes attained through the use of the methods.
Introduction
De novo protein design is the identification of protein sequences that will yield a desired tertiary structure with improved properties or function. Since the native fold of a protein is the conformation which lies at the free energy minimum, de novo protein design seeks sequences that will have a free energy minimum in the target fold. This problem was first described by Drexler1 and Pabo2 and was referred to as the "inverse folding problem." However, unlike the protein folding problem, where a sequence can yield only one folded structure solution, the de novo protein design problem exhibits degeneracy. Many different amino acid sequences can yield the same tertiary structure and function.
While protein design has traditionally been performed experimentally through rational design and directed evolution, computational methods have more recently been employed to overcome the limited search space inherent in experimental methods. A variety of computational methods have been used, including deterministic methods, stochastic methods, and probabilistic methods.3,4 Early computational methods used fixed-backbone templates to make the problem easier to solve.5-7 With the advent of faster processors, high performance computing, and more efficient algorithms, backbone flexibility has been incorporated by using an ensemble of fixed-backbone templates8-14 or by incorporating true backbone flexibility by expressing the template in terms of ranges of atom-to-atom distances and dihedral angles.15,16
This paper describes in detail Protein WISDOM, an online tool that has been made available to the academic community to utilize our computational de novo protein design framework. This framework has been applied to the design of numerous proteins, for therapeutic use targeting diseases such as HIV, cancer, complement diseases, and other autoimmune disorders. Many of the predicted peptides were experimentally validated, demonstrating the power of the method. Table 1 provides a summary of the different proteins that have been designed including the size of the protein or peptide, the number of predictions, and experimental validation.
| Protein Design | Protein Length | # of Computational Predictions | # of Experimental Validations | Reference |
| Full sequence design of human beta-defensin-2 | 41 | 340 | (17) | |
| Compstatin inhibitors of human C3 | 13 | 28 | 3/3 | (18, 19) |
| Compstatin analogues that bind to rat C3c | 13 | 5 | (20) | |
| Compstatin analogues with di-serine extension | 15 | 8 | ||
| Stabilizing structure of compstatin analog W4A9 | 13 | 18 | ||
| C3a receptor agonists and antagonists | 77 | 20 | 4/7 | (21) |
| C5a receptor agonists and antagonists | 74 | 61 | 2/61 | |
| HIV-1 gp14 inhibitors | 12 | 6 | 4/5 | (22) |
| HIV-1 gp120 inhibitors | 9 | 14 | ||
| Bak inhibitors of Bcl-x L and Bcl-2 | 16-18 | 10 | 5/5 | (23) |
| Inhibitors of ERK2 | 11 | 25 | ||
| Inhibitors of EZH2 | 21 | 17 | 10/10 | (24) |
| Inhibitors of LSD1 and LSD2 | 16 | 41 | 17/20 | |
| Inhibitors of HLA-DR1 | 13 | 6 | (25) | |
| Inhibitors of PNP | 5 | 13 |
Table 1. Summary of designed proteins and peptides using the de novo protein design framework. The # of computational predictions is presented as the number of favorable predictions (i.e. fold specificities above a certain cutoff or approximate binding affinities greater than the native sequence). The # of experimental validations gives two numbers: the first is the number of predictions that were experimentally validated while the second is the total number of predictions that were tested experimentally.
Design of human-beta-defensin-2 (hβD-2) was performed to enhance the peptide's antimicrobial property.17 For this design, we considered two cases: 1) up to 10 mutations along hβD-2 and 2) full sequence design of all hβD-2 residue positions except the Cysteines (8, 15, 20, 30, 37, and 38). Three different design templates and three different sequence selection models were utilized in the design. High levels of similarity in mutations were observed between the weighted average and distance bin models for both the 10 mutation design and the full sequence design. Additionally, a large number of sequences were found to have more favorable calculated Fold Specificity values than the native sequence.
Complement system inhibitors (of C3, C3a, and C5a) were designed to combat a number of immune diseases such as stroke, heart attack, Alzheimer's disease, asthma, rheumatoid arthritis, rejection of xenotransplantation, adult respiratory disease, psoriasis, and Crohn's disease. Three compstatin inhibitors of C3c predicted by the protein design framework plus three rationally designed sequences were experimentally validated to be better binders than the native compstatin.18,19
Further studies examined the loss of activity of compstatin against non-primate C3c and designed a number of candidate rat and mouse C3c inhibitors. Five sequences were shown to have more favorable association free energies with rat C3c than the W4A9 compstatin mutant known to inhibit C3c. This is due to a new salt bridge formation by Arg1.20 Eight sequences with an N-terminal extension were predicted to be better binders than W4A9 with a di-Serine extension. Finally, 18 compstatin sequences were predicted to stabilize the bound conformation of W4A9, providing strong candidates for primate and non-primate C3c inhibitors.
In addition to C3c inhibitors, C3a and C5a receptor agonists and antagonists were designed based upon the structures of C3a and C5a. Seven C3a sequences predicted by the model were experimentally tested. Two of the sequences were potent agonists while two others were partial agonists.21 The two potent agonists showed a 58-fold improvement over a previously discovered "superagonist". The design of C5a receptor agonists and antagonists provided a set of 61 sequences. All the sequences were synthesized and two were found to be novel C5a agonists.
Fusion inhibitors of HIV-1, the virus that causes AIDS, were designed to prevent HIV-1 from infecting cells. The first design targeted gp41, an envelope glycoprotein of HIV-1. The protein design framework predicted six sequences that were better binders than the native sequence. Four of these predicted sequences were experimentally validated to inhibit HIV-1 with the best sequence having an IC50 as low as 29 μM. This sequence showed a 3-15 fold improvement over the native sequence and had no loss of activity against an Enfuvirtide-resistant virus strain.22 The second design targeted gp120, another envelope glycoprotein of HIV-1. Fourteen sequences were predicted to be binders of gp120 and provide additional potential fusion inhibitors of HIV-1.
Numerous proteins linked to cancer provided promising targets for cancer therapeutics. Bcl-2 and Bcl-xL are anti-apoptotic proteins that prevent cell death. Inhibitors of these two proteins were designed to induce cell death in cancer cells. Ten sequences were predicted to be better binders than the native, and these results captured previous experimental and mutagenesis results.23 Another target protein, ERK2, is involved in signal-transduction cascades that make it a promising target for antiproliferative cancer therapies. Twenty-five sequences were predicted to be inhibitors of ERK2.
Histone methyltransferases and demethylases dynamically control histone methylation, which has been linked to many cancer types including prostate, breast, lymphoma, myeloma, bladder, colon, skin, liver, endometrial, lung, and gastric. The de novo protein design framework identified 17 inhibitors of EZH2 (a Lysine methyltransferase) and of the ten experimentally tested, all were found to inhibit EZH2.24 The most potent peptide had an IC50 of about 13 μM, was equally effective with elevated enzyme concentrations, and did not compete with the cofactor. These peptides were the first set of inhibitors of EZH2. 53 inhibitors of LSD1 (a demethylase) were predicted by the framework and of the 20 experimentally tested, 17 were inhibitors of LSD1 and 18 were inhibitors of LSD2. The best inhibitors had IC50 values below 1 μM, making them the most potent peptidic inhibitors discovered to date.
The final two protein systems provided targets for treating various autoimmune diseases such as Coeliac disease, diabetes mellitus type 1, systemic lupus erythematosus, Sjögren's syndrome, Churg-Strauss Syndrome, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, rheumatoid arthritis, and allergies. None of these potential inhibitors have been experimentally validated, however the framework predicted six sequences that bind to HLA-DR1 and 13 sequences that bind to PNP.
Table 2 summarizes experimentally validated inhibitors and agonists predicted using the de novo protein design framework. The approximate binding affinity metric was used to predict nine of the sequences (inhibitors of human C3c, HIV-1 gp41, EZH2, LSD1, and LSD2), while the fold specificity metric was used to identify four of the sequences (agonists/antagonists of C3aR). These peptides highlight the success of the de novo protein design framework, particularly the added approximate binding affinity metric. The framework is extremely versatile in its applicability. Six different proteins linked to twenty-five different diseases have been successfully designed and experimentally validated.
| Name | IC50 | EC50 | Protein Target | Applicable Diseases |
| SQ027 | 0.94 μM | human C3c | stroke, heart attack, Alzheimer's disease, asthma, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, psoriasis, diabetes type I, Crohn's disease, pancreatitis, and cystic fibrosis | |
| SQ086 | 1.98 μM | human C3c | ||
| SQ059 | 4.73 μM | human C3c | ||
| SQ110-4 | 15.2 nM | C3aR | ||
| SQ060-4 | 36.4 nM | C3aR | ||
| SQ007-5 | 15.4 nM | C3aR | ||
| SQ002-5 | 26.1 nM | C3aR | ||
| SQ435 | 29 - 253 μM | HIV-1 gp41 | AIDS | |
| SQ037 | 13.57 μM | EZH2 | prostate, breast, lymphoma, myeloma, bladder, colon, skin, liver, endometrial, lung, and gastric cancers | |
| SQ011-1 | 0.521 μM | LSD1 | ||
| SQ016-1 | 0.249 μM | LSD1 | ||
| SQ026-1 | 2.51 μM | LSD2 | ||
| SQ015-1 | 1.332 μM | LSD2 |
Table 2. Computationally predicted and experimentally validated peptides targeting various diseases.
Protocol
Method Overview
The de novo design framework used in Protein WISDOM consists of two stages. The first stage produces a rank-ordered list of amino acid sequences that will fold into a given template structure. The second stage validated these sequences by calculating either fold specificity or approximate binding affinity, or both. The former is primarily used when the design is of a single protein, while the latter is used when the design is of a complex (a peptide binding to a target protein). Figure 1 gives an overview of the steps involved in the framework.
Design Inputs: A number of inputs need to be defined for the de novo protein design framework. The first is the design template. This is a 3-dimensional (3D) protein structure that contains coordinates for all the atoms in the protein. The structure can be rigid or flexible. Rigid templates are a set of fixed atom coordinates and are obtained from x-ray crystallography structures. Flexible templates can be a set of fixed atom coordinates or upper and lower bounds on the atom coordinates. These templates can be obtained from NMR solution structures, molecular dynamics, or docking simulations.
The design template is used to generate the allowed mutation set of the designed protein. This set defines which positions of the sequence can mutate and to what amino acids. The mutation set is generated by calculating the solvent accessible surface area (SASA) of each residue in the design template. If the residue is more than 50% exposed to solvent, a set of hydrophilic amino acids is allowed (D, E, G, H, K, N, P, Q, R, S, T). If the residue is less than 20% exposed to solvent, a set of hydrophobic amino acids is allowed (A, F, I, L, M, V, W, Y). If the residue's exposure is in between 20% and 50%, all amino acids are allowed. Cysteine is typically excluded from the mutation set unless experimental or literature data deem it appropriate. The small amino acids (A, G, T) are typically included in all mutation sets. When available, experimental or literature insights can be used to manually modify the mutation sets of particular amino acid positions.
A forcefield is chosen to calculate the pairwise interaction energy of the sequences in the design template. While any forcefield can be adapted to be used within the framework, two distance-dependent forcefields have been developed and are used extensively in the de novo design framework. The first is a high resolution Cα-Cα forcefield,26 where the distances are between the Cα carbons of the residues. The second is a high resolution centroid-centroid forcefield27 where the distances are between the centroids of the residues. The energy parameters in the forcefields were derived by solving a linear programming parameter estimation problem which required the low-energy high-resolution decoys for a large training set of proteins to be energetically less favorable than their native conformations. The high-resolution centroid-centroid forcefield and the Cα-Cα forcefield were both tested and validated in previous studies on human beta-defensin-2.17 True backbone flexibility is incorporated into the model by discretizing the forcefields into distance bins. The distance between a pair of amino acids will correspond to a distance bin giving the same energy value to a range of distances. This enables the sequence selection optimization model to account for backbone movement.
Biological constraints, in the form of charge constraints or content constraints, can be included manually by the user as an additional design input. Charge constraints specify a particular charge or range of charges that must be satisfied for the designed sequence or a portion of the designed sequence. The charge is calculated as the sum of the positively charged residues (K and R) minus the sum of the negatively charged residues (D and E). Content constraints specify upper and lower bounds on the occurrence of a particular amino acid in the sequence. Biological Constraints are generally defined through an extensive sequence alignment to the native sequence. This is to capture the known biological limits on charge and amino acid content represented in nature for a family of proteins. Further constraints are manually defined through analysis of known experimental data.
Stage One: Sequence Selection: The original sequence selection method was first developed by Klepeis et al.15,16 It selects and ranks amino acid sequences according to their energies in the design template using an Integer Linear Optimization (ILP) model. The method was later improved by the use of a more computationally efficient sequence selection model for rigid (single) templates and expanded through the development of models for flexible templates. This global optimization method does not rely on random mutations and is theoretically guaranteed to search the complete sequence space and determine a global solution. This is a major advantage of our approach compared to all other existing approaches.
Single Structure Model: The original form of the sequence selection model proposed by Klepeis et al.15,16 was further refined by Fung et al.28 Its final form is given in Eq. 1.
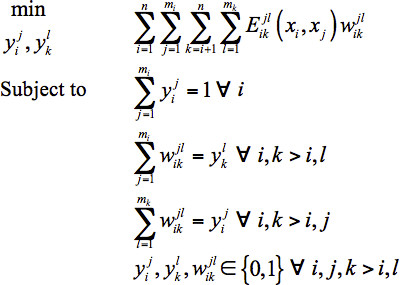
Set i=1,...,n defines the residue positions in the design template. At each position i, mutations are represented by j{i}=1,...,mi, where mi=20 if position i is allowed to mutate to any of the twenty natural amino acids. The alias sets k≡i and l≡j, with k>i, are employed to represent all unique pairwise interactions. Binary variables 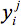 and
and 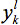 are introduced to model amino acid mutations. The
are introduced to model amino acid mutations. The 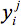 variable will assume the value of one if the model assigns amino acid j to position i, and the value of zero otherwise (similarly for
variable will assume the value of one if the model assigns amino acid j to position i, and the value of zero otherwise (similarly for 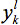 ). The objective function represents the sum of all pairwise energy interactions in the design template. Parameter
). The objective function represents the sum of all pairwise energy interactions in the design template. Parameter 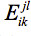 which is the energy interaction between position i occupied by amino acid j and position i occupied by amino acid l , depends on the distance between the α-carbons or side chain centroids at the two positions (xi,xj,) as well as the type of amino acids j and l . It only contributes to the objective function if both
which is the energy interaction between position i occupied by amino acid j and position i occupied by amino acid l , depends on the distance between the α-carbons or side chain centroids at the two positions (xi,xj,) as well as the type of amino acids j and l . It only contributes to the objective function if both 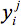 and
and 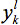 are equal to one.
are equal to one.
Fung et al.28 found that formulation (1) is significantly more computationally efficient than twelve other equivalent quadratic assignment-like models for sequence selection.28,29 In particular, it outperformed the original model proposed by Klepeis et al.15,16 on two sequence selection problems for human beta-defensin-2: one at a complexity level of 3.4x1045 and the other at 6.4x1037 with 49 additional linear biological constraints. The original model proposed by Klepeis et al.15,16 was found to take 53,263 central processing unit (CPU) sec and 4,578 CPU sec respectively to solve the two problems to global optimality using CPLEX 9.030 on a Pentium IV 3.2 GHz processor. Formulation (1) only took 649 CPU sec and 14 CPU sec to perform the same tasks, corresponding to an 82-fold and 327-fold improvement in computational efficiency.
Weighted Average Model: Fung et al.28 developed two models to handle the typical case of de novo protein design in which the design template is flexible, containing a set of structures. The Weighted Average Model uses a weighted average energy, 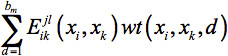 , in place of the energy parameter
, in place of the energy parameter 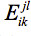 (xi,xk) in the Single Structure Model (Eq. 1). The weights wt(xi,xk,d) are determined by the frequencies of the distance between xi and xk falling into distance bin d in the template structures. The final form of the Weighted Average Model is given in Eq. 2.
(xi,xk) in the Single Structure Model (Eq. 1). The weights wt(xi,xk,d) are determined by the frequencies of the distance between xi and xk falling into distance bin d in the template structures. The final form of the Weighted Average Model is given in Eq. 2.
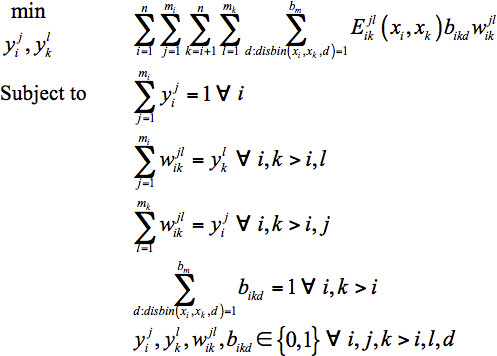
Distance Bin Model: The second sequence selection model for flexible template structures incorporates the distance information from the multiple structures by introducing a binary variable bikd. This variable equals one if the distance between xi and xk falls into distance bin d , and is zero otherwise. Another parameter introduced, disbin(xi, xk, d) , equals one if the distance between xi and xk in any of the template structures falls into distance bin d and is zero otherwise. Since only one distance bin per amino acid pair will contribute to the total energy,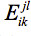 in the objective function is replaced with
in the objective function is replaced with 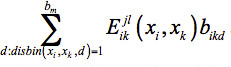 . This, however, introduces nonlinearity into the objective function. Further details on linearizing the model and additional constraints that need to be added for feasibility can be found in Fung et al.28 The Distance Bin Model is given in Eq. 3.
. This, however, introduces nonlinearity into the objective function. Further details on linearizing the model and additional constraints that need to be added for feasibility can be found in Fung et al.28 The Distance Bin Model is given in Eq. 3.
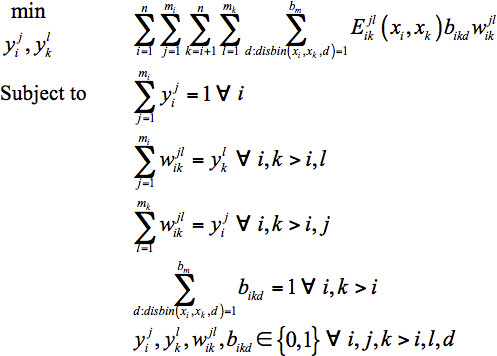
Any of the above formulated Integer Linear Programming (ILP) problems15-17 can be solved rigorously using branch-and-bound techniques.28-30 Such techniques guarantee consistent and reliable convergence to the global minimum energy sequence.
Stage Two: Validation: Figure 2 provides a detailed overview of the two Stage Two approaches. The figure shows the steps required to calculate the final ranking metric and the number of structures generated in each step.
Fold Specificity: Fold specificity is a metric used for ranking preliminary designs derived in Stage One. The aim of the calculation is to find how well each sequence folds into the template structure relative to the original sequence of the template, based on energy calculations. There are two approaches for how to do this, each with different computational demands.
The first approach was implemented by Klepeis et al.15,16 This approach utilizes the protein structure prediction framework ASTRO-FOLD, 26,27,31-47 which is based on deterministic global optimization. This approach is not currently used in the implementation of Protein WISDOM since it is very computationally demanding. Recognizing computational resource limitations and the need to perform this calculation on potentially hundreds to thousands of sequences in design, Fung et al.17 proposed a more efficient approach using TINKER/CYANA.48-50 The approach involves defining a flexible template of the structure. The flexible template can be defined using upper and lower bounds on the distances between Cα atoms, as well as the ϕ and ψ angles of the residues. For a single structure, the initial distances and dihedral angles are used and bounds are defined either as a fixed distance or a percentage. The default bounds are ±10% for Cα distances or ±10° for dihedral angle bounds. For a flexible template, bounds can be obtained from the maximum and minimum values seen across all template structures given as input to design. Once initial bounds are defined for each sequence, ensembles containing hundreds of conformers are generated using CYANA 2.1.48,49The conformers are generated using a torsion angle dynamics simulated annealing protocol in CYANA that heats the protein rapidly and slowly cools it, tracking the conformations sampled. After the simulated annealing, a local energy minimization is performed that minimizes the clashes from Van der Waals radii overlapping, as well as violations in the distance and angle constraints. By default, 500 final structures are generated. Each structure in the ensemble for each sequence is subjected to a local minimization in TINKER 3.6,50 using the AMBER forcefield.51 The final potential energy of each minimized structure is tabulated. This overall approach is performed for the starting sequence as well as each candidate mutant sequence. Then, the Fold Specificity of each mutant sequence to the target fold can be calculated relative to the native sequence using the following Boltzmann distribution (Eq. 4).
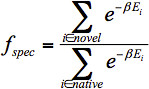
Approximate Binding Affinity: The approximate binding affinity calculation method is used to rank the designed sequences that are in complex with a target protein. These calculations can be done on the sequences directly from Stage One or can be performed on the high fold specificity sequences obtained from the fold specificity step.
Lilien et al.52 proposed an approach for the calculation of approximate binding affinities of protein-ligand complexes. It is based on generating rotamerically-based ensembles of the protein, the ligand, and the protein-ligand complex and using those ensembles to calculate partition functions. This approximate binding affinity is denoted as K* and is defined by Eq. 5.
Here qPL is the partition function of the protein-ligand complex, qb is the partition function of the free protein, and qL is the partition function of the free ligand. The partition functions are defined in Eq. 6, where the sets B, F and L contain the rotamerically-based conformations of the bound protein-ligand complex, the free protein, and the free ligand, respectively. En is the energy of conformation n, R is the gas constant, and T is the temperature.
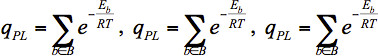
Structure Prediction: In order to begin calculating K* , a 3D structure of each sequence is needed. This is done using the Rosetta AbRelax function,53-55 part of the Rosetta 3.4 software package. The strategy behind the AbRelax algorithm is based upon experimental observation that the local structure of the protein is influenced but not uniquely determined by the local sequence of the protein. A Monte Carlo algorithm is used to replace local protein structures with sequence derived structural fragments. This method produces the final compact protein structures that account for non-local interactions such as buried hydrophobic residues, paired β strands, and specific side chain interactions.
Clustering: The structures from AbRelax are then clustered based upon their φ and ψ angles using OREO.56,57 This clustering method elucidates representative backbone structures of the entire structural ensemble. The average structures from the ten largest clusters and the overall lowest energy structure are chosen for docking to the target protein. This provides 11 unique backbone structures for each peptide sequence, incorporating backbone flexibility into the ensemble generation.
Docking Prediction: Docking prediction is done using RosettaDock.58-60 For each sequence, each of the 11 peptide backbone structures is docked against the target protein. In this case, since the binding site is known, the peptides are placed near the binding site and allowed to translate 3 Å normal to the binding site, 8 Å parallel to the binding site, and rotate 8°. RosettaDock uses a Monte Carlo algorithm for low and high resolution docking movements. Each docking run generates a large ensemble of complex structures. The ten lowest energy complexes in each of the 11 runs are used as starting structures in the final rotamerically-based conformation ensemble generation (110 starting structures per sequence).
Final Ensemble Generation: RosettaDesign61 is used to generate the final rotamerically-based conformation ensemble because it can be used to generate a number of structures by only adjusting the rotamers on the side chains through the fixbb function. RosettaDesign is given a number of starting structures, and for each structure, a residue is randomly chosen and the rotamer changed through a Monte Carlo algorithm. This is repeated until thousands of rotamer substitutions are attempted and gives a final low-energy conformation that will contribute highly to the partition function.
To generate the peptide ensemble, the ten lowest-energy peptide structures from each of the ten largest clusters plus the ten overall lowest-energy peptide structures are used as starting structures for RosettaDesign (110 total starting structures). For each starting structure, 200 rotamer conformers are generated, giving a final ensemble of 22,000 structures (set L in Eq. 6). The ensemble incorporates both backbone flexibility and rotamer flexibility.
The complex ensemble is generated similarly by taking the 110 starting structures from the docking prediction step and generating 200 rotamer conformers per starting structure. The final ensemble size is 22,000 structures (set B in Eq. 6). Flexibility is taken into account by the various peptide backbone structures used, the various docked conformations, and the rotamer conformers for each starting structure.
The protein ensemble is generated by running RosettaDesign on just the target protein structure. In this case, 2,000 rotamer conformations are generated for the single starting structure, so the final ensemble size is 2,000 structures (set F in Eq. 6).
Protein WISDOM
Protein WISDOM, which stands for Protein Workbench for In Silico De novo design Of bioMolecules, is an online tool that gives the academic community access to our de novo protein design framework in a user-friendly way. It can handle several commonly encountered design objectives, from designing single protein chains to adopt a template fold to designing novel peptides that will bind to a target protein. The next two sections describe the capabilities of Protein WISDOM with regards to the two main types of protein design problems encountered. The first type applies sequence selection to select novel sequences that are favorable in the given design template and then uses fold specificity to validate the novel sequences. The second type uses sequence selection to select novel sequences of a peptide bound in a complex and then uses both fold specificity and approximate binding affinity calculations to validate the novel sequences.
User Registration
Visit the Protein WISDOM web page at http://www.proteinwisdom.org.
Click the User Login button on the top right of the page. Click the "Click here" to register.
Fill out information related to email address and requested username and click continue.
Fill out additional information on name, institution, group, address. Click the checkbox to agree to terms of use. Click the "Submit Registration" button.
Stage One: Sequence Selection
Submission of Protein Sequence and Template Structure(s)
Click on the User Login button to begin the protein design experiment. The user is presented with their "User Homepage" (Figure 3) which lists the number of jobs they have submitted, the number of structures (templates) they have uploaded, and a list of the structures they have uploaded so far.
Start a new design job by clicking "Create New Job." The user is taken to the "Job Submission" page (Figure 4). Give the job a name, and indicate if it is based on a previous job (i.e. the same design template, mutation sets, and biological constrains can be imported into a new job, however the user will have the ability to modify the mutation sets and biological constraints). Click "continue."
Upload the protein structure(s) of the design template (Figure 5). This template must be in standard protein data bank (PDB) format. It can be a rigid template (one set of coordinates for every atom) or a flexible template (multiple models, such as obtained from NMR solution structures). For the case of designing a single protein, there can only be one chain in the template. A user can upload a new template or select from existing templates they have previously uploaded. Optionally indicate the pdb ID of the template, if available. If multiple templates are uploaded, be sure each model begins with "MODEL #" and ends with "ENDMDL." Ensure every residue is designated by a natural amino acid. Click "Continue."
Upon successful upload of the template, Protein WISDOM will display the number of residues, chains, and models it found in the template, list the sequence, and ask the user to verify the template. Confirm the template structure if it has been correctly inputted, and click "Continue."
Once the template has been successfully uploaded and confirmed, the user is taken to the "Main Control Page" (Figure 6). On this page, the user can view the job status, modify the mutation sets and biological constraints, and submit the job for Stage One: Sequence Selection. At this point, since Stage One has not completed, there are no options for Stage Two. Those appear once results from Stage One are available.
Selection of Mutation Sets
Click on the "Mutation Sets" link on the "Main Control Page" to define mutation sets.
Select which residues will be allowed to mutate, and select which amino acids they are allowed to mutate to (Figure 7). By default, the allowable amino acids at any given position are selected based upon Solvent Accessible Surface Area (SASA). Mutation sets are required.
Click "Save Changes" after mutation sets are selected. The user can choose to continue editing the mutation set. When finished editing the mutation set, click to return back to the "Main Control Page."
Selection of Biological Constraints
Click on the "Biological Constraints" link on the "Main Control Page" to define biological constraints.
Specify charge or amino acid content constraints across the whole protein or a portion of the protein (Figure 8).
Limit the total number of mutations allowed to occur, if required. Biological constraints are optional. Click to return to the "Main Control Page" when finished.
Submission of Stage One: Sequence Selection
Click on the "Begin Stage 1" link to bring user to "Submit Stage 1" page.
Select the chain to design (Figure 9), the number of sequences to generate, the distance-dependent forcefield, and the model. If a complex is being design and a Fold Specificity calculation is desired, one must choose only a single chain to design. If the uploaded template was a single structure, or a "rigid template," only the Single Structure model is allowed. If the uploaded template is flexible, the user has the option to select from all three models: Single Structure, Weighted Average, and Distance Bin. Take note of the computational complexity of the optimization to be solved. There is an upper limit of 2025 for computational complexity allowed.
Submit the job. The user is redirected back to the "Main Control Page" (Figure 10). The Job Status will be updated to indicate the current progress of the job. The job will become locked for editing after submission.
Upon completion of the job, the user receives an email with the results, which consist of a list of designed sequences. The results are also viewable on the "Main Control Page." A box for Stage 2: Fold Specificity appears on the page to enable the user to perform this validation.
Stage Two: Fold Specificity Calculations
Fold Specificity Submission
Click "Begin Stage 2: Fold Specificity" to enter the "Build Stage 2" page. Define the upper and lower Cα-Cα distance bounds by specifying the Template flexibility factor either as a percentage of distance, or as a fixed distance. Define upper and lower angle bounds on the φ and ψ dihedral angles by specifying the Template flexibility factor as a percentage. Note that when using a flexible template, the upper and lower distance bounds are taken as the lowest and highest distance values across all the template models. Likewise, upper and lower angle bounds are taken from the highest and lowest angle values across all the models.
Click the "Submit" button.
Specify the number of structures per sequence to generate and click "Continue." Note there is an upper bound of 500 structures per sequence to generate.
Click "Continue" to confirm intent to submit for fold validation. Stage One and Stage Two are locked for editing until the completion of Stage Two.
Upon completion of the job, an email is sent to the user with the results. View the results on Protein WISDOM on the "Main Control Page" (Figure 11). Here the text files containing designed sequences, corresponding energy values from Stage One and fold specificity values from Stage Two can be viewed and downloaded. In addition, the user may click the "View Results" link which displays a table in the browser with Stage One ranks and energy values as well as Stage Two ranks and fold specificity values.
Stage Three: Approximate Binding Affinity Calculations for Protein-peptide Complexes
Approximate Binding Affinity calculations calculate the affinity of the designed ligand protein/peptide to the rest of the complex. These calculations can be performed directly after Stage One, or after Fold Specificity calculations have been completed.
Click on "Sequence #" to select the sequence to begin approximate binding affinity calculation. User will be directed to the "Select Sequence" page, which presents a list of the designed sequences along with their sequence selection and fold specificity ranks. Only one sequence can be selected at a time for approximate binding affinity calculation, as the calculations are very computationally demanding. Upon completion of a sequence, the user may select another sequence to have the approximate binding affinity calculated, and this result is added to the previous result, displaying the approximate binding affinity for all completed sequences. Once a sequence is selected and saved, the user is redirected to the "Main Control Page."
Click "Begin Stage 2: Approximate Binding Affinity" to submit the job. Upon completion, results are emailed to the user, which include an attachment containing the sequence number, approximate binding affinity, and values of the partition functions in Eq. 6. For every subsequent approximate binding affinity job, this file contains the results for all the completed sequences. Full results (from sequence selection, fold specificity, and approximate binding affinity) can also be viewed by accessing the "Main Control Page" for the job (Figure 12).
Results
De Novo Design of Entry Inhibitors for HIV-1
The de novo design framework implemented in Protein WISDOM has been used for the design of inhibitor peptides for several important therapeutic systems (Tables 1 and 2). One system of note is the design of peptides to inhibit HIV-1 entry to the host cell receptor CD4, which is here used as a representative system to demonstrate the practical use of the Protein WISDOM interface. The peptides were designed to target the...
Discussion
The de novo protein design framework consists of two stages, a sequence selection stage and a validation stage. The framework is robust enough to handle rigid and flexible design templates, and can be applied to single protein design or complex protein design. The framework has been successfully applied to numerous protein systems with applications to dozens of diseases. A number of the designs have been experimentally validated, providing the most potent inhibitors or agonists of some proteins discovered to dat...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
CAF gratefully acknowledges support from NSF, NIH (R01 GM52032; R24 GM069 736), and the US Environmental Protection Agency, EPA (R 832721-010). A portion of this research was made possible with Government support by DoD, Air Force Office of Scientific Research. JS gratefully acknowledges support from NIH (P50GM071508-06). MLBP gratefully acknowledges support from a National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. GAK gratefully acknowledges support from a National Science Foundation Graduate Research Fellowship under grant number DGE-1148900.
References
- Drexler, K. Molecular engineering: An approach to the development of general capabilities for molecular manipulation. Proc. Natl Acad. Sci. U.S.A. 78, 5275-5278 (1981).
- Pabo, C. Molecular technology: Designing proteins and peptides. Nature. 301, 200 (1983).
- Floudas, C. A. Research challenges, opportunities and synergism in systems engineering and computational biology. AIChE J. 51, 1872-1884 (2005).
- Fung, H. K., Welsh, W. J., Floudas, C. A. Computational de novo peptide and protein design: Rigid templates versus flexible templates. Ind. Eng. Chem. Res. 47, 993-1001 (2008).
- Ponder, J., Richards, F. Tertiary templates for proteins. J. Mol. Biol. 193, 775-791 (1987).
- Dahiyat, B. I., Mayo, S. L. Protein design automation. Protein Sci. 5, 895-903 (1996).
- Dahiyat, B. I., Gordon, D. B., Mayo, S. L. Automated design of the surface positions of protein helices. Protein Sci. 6, 1333-1337 (1997).
- Su, A., Mayo, S. L. Coupling backbone flexibility and amino acid sequence selection in protein design. Protein Sci. 6, 1701-1707 (1997).
- Desjarlais, J., Handel, T. Side chain and backbone flexibility in protein core design. J. Mol. Biol. 290, 305-318 (1999).
- Farinas, E., Regan, L. The de novo design of a rubredoxin-like Fe site. Protein Sci. 7, 1939-1946 (1998).
- Harbury, P. B., Plecs, J. J., Tidor, B., Alber, T., Kim, P. S. High-resolution protein design with backbone freedom. Science. 282, 1462-1467 (1998).
- Koehl, P., Levitt, M. De novo protein design: I. In search of stability and specificity. J. Mol. Biol. 293, 1161-1181 (1999).
- Koehl, P., Levitt, M. De novo protein design. II. Plasticity in sequence space. J. Mol. Biol. 293, 1183-1193 (1999).
- Kuhlman, B., Dantae, G., Ireton, G., Verani, G., Stoddard, B., Baker, D. Design of a novel globular protein fold with atomic-level accuracy. Science. 302, 1364-1368 (2003).
- Klepeis, J. L., Floudas, C. A. Integrated structural, computational and experimental approach for lead optimization: Design of compstatin variants with improved activity. J. Am. Chem. Soc. 125, 8422-8423 (2003).
- Klepeis, J. L., Floudas, C. A., Morikis, D., Tsokos, C. G., Lambris, J. D. Design of peptide analogs with improved activity using a novel de novo protein design approach. Ind. Eng. Chem. Res. 43, 3817-3826 (2004).
- Fung, H. K., Floudas, C. A., Taylor, M. S., Zhang, L., Morikis, D. Toward full-sequence de novo protein design with flexible templates for human beta-defensin-2. Biophys. J. 94, 584-599 (2008).
- Bellows, M. L., Fung, H. K., Floudas, C. A., López de Victoria, A., Morikis, D. New compstatin variants through two de novo protein design frameworks. Biophys. J. 98, 2337-2346 (2010).
- López de Victoria, A., Gorham, R. D. A new generation of potent complement inhibitors of the compstatin family. Chem. Biol. Drug Des. 77, 431-440 (2011).
- Tamamis, P., López de Victoria, A. Molecular dynamics in drug design: New generations of compstatin analogs. Chem. Biol. Drug Des. 79, 703-718 (2012).
- Bellows-Peterson, M. L., Fung, H. K. De novo peptide design with c3a receptor agonist and antagonist activities: Theoretical predictions and experimental validation. J. Med. Chem. 55, 4159-4168 (2012).
- Bellows, M. L., Taylor, M. S. Discovery of entry inhibitors for HIV-1 via a new de novo protein design framework. Biophys. J. 99, 3445-3453 (2010).
- Sun, J. -. J., Abdeljabbar, D. M., Clarke, N. L., Bellows, M. L., Floudas, C. A., Link, A. J. Reconstitution and engineering of apoptotic protein interactions on the bacterial cell surface. J. Mol. Biol. 394, 297-305 (2009).
- Smadbeck, J., Bellows-Peterson, M. L. De novo protein design and validation of histone methyltranferase inhibitors. , (2013).
- Bellows, M. L., Fung, H. K., Floudas, C. A., Adjiman, C. S., Galindo, A. . Molecular Systems Engineering, Process Systems Engineering. 6, 207-232 (2010).
- Rajgaria, R., McAllister, S. R., Floudas, C. A. A novel high resolution Cα-Cα distance dependent force field based on a high quality decoy set. Proteins. 65, 726-741 (2006).
- Rajgaria, R., McAllister, S. R., Floudas, C. A. Distance dependent centroid to centroid force fields using high resolution decoys. Proteins. 70, 950-970 (2008).
- Fung, H. K., Taylor, M. S., Floudas, C. A. Novel formulations for the sequence selection problem in de novo protein design with flexible templates. Optim. Method. Softw. 22, 51-71 (2007).
- Fung, H. K., Rao, S., Floudas, C. A., Prokopyev, O., Pardalos, P. M., Rendl, F. Computational comparison studies of quadratic assignment like formulations for the in silico sequence selection problem in de novo protein design. J. Comb. Optim. 10, 41-60 (2005).
- CPLEX. . Using the CPLEX Callable Library. , (1997).
- Klepeis, J. L., Floudas, C. A. Free energy calculations for peptides via deterministic global optimization. J. Chem. Phys. 110, 7491-7512 (1999).
- Klepeis, J. L., Floudas, C. A., Morikis, D., Lambris, J. D. Predicting peptide structures using NMR data and deterministic global optimization. J. Comput. Chem. 20, 1354-1370 (1999).
- Klepeis, J. L., Schafroth, H. D., Westerberg, K. M., Floudas, C. A. Deterministic global optimization and ab initio approaches for the structure prediction of polypeptides, dynamics of protein folding and protein-protein interactions. Adv. Chem. Phys. 120, 265-457 (2002).
- Klepeis, J. L., Floudas, C. A. Ab initio prediction of helical segments of polypeptides. J. Comput. Chem. 23, 246-266 (2002).
- Klepeis, J. L., Floudas, C. A. Prediction of beta-sheet topology and disulfide bridges in polypeptides. J. Comput. Chem. 24, 191-208 (2003).
- Klepeis, J. L., Floudas, C. A. ASTRO-FOLD: A combinatorial and global optimization framework for ab initio prediction of three-dimensional structures of proteins from the amino acid sequence. Biophys. J. 85, 2119-2146 (2003).
- Klepeis, J. L., Pieja, M. T., Floudas, C. A. A new class of hybrid global optimization algorithms for peptide structure prediction: Integrated hybrids. Comput. Phys. Commun. 151, 121-140 (2003).
- Klepeis, J., Pieja, M., Floudas, C. Hybrid global optimization algorithms for protein structure prediction : Alternating hybrids. Biophys. J. 84, 869-882 (2003).
- Klepeis, J. L., Floudas, C. Analysis and prediction of loop segments in protein structures. Comput. Chem. Eng. 29, 423-436 (2005).
- Mo¨nnigmann, M., Floudas, C. Protein loop structure prediction with flexible stem geometries. Proteins. 61, 748-762 (2005).
- McAllister, S. R., Mickus, B. E., Klepeis, J. L., Floudas, C. A. A novel approach for alpha-helical topology prediction in globular proteins: Generation of interhelical restraints. Proteins. 65, 930-952 (2006).
- Floudas, C. A., Fung, H. K., McAllister, S. R., Mönnigmann, M., Rajgaria, R. Advances in protein structure prediction and de novo protein design: A review. Chem. Eng. Sci. 61, 966-988 (2006).
- Subramani, A., Wei, Y., Floudas, C. A. ASTRO-FOLD 2.0: An enhanced framework for protein structure prediction. AIChE J. 58, 1619-1637 (2012).
- Wei, Y., Thompson, J., Floudas, C. Concord: a consensus method for protein secondary structure prediction via mixed integer linear optimization. P. Roy. Soc. A-Math. Phy. 468, 831-850 (2011).
- Subramani, A., Floudas, C. β-sheet topology prediction with high precision and recall for β and mixed α/β proteins. PLoS One. 7, e32461 (2012).
- Rajgaria, R., Wei, Y., Floudas, C. A. Contact prediction for beta and alpha-beta proteins using integer linear optimization and its impact on the first principles 3D structure prediction method ASTRO-FOLD. Proteins. 78, 1825-1846 (2010).
- Subramani, A., Floudas, C. A. Structure prediction of loops with fixed and flexible stems. J. Phys. Chem. B. 116, 6670-6682 (2012).
- Güntert, P., Mumenthaler, C., Wüthrich, K. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273, 283-298 (1997).
- Güntert, P. Automated NMR structure calculation with CYANA. Methods Mol. Biol. 278, 353-378 (2004).
- Ponder, J. TINKER, software tools for molecular design. , (1998).
- Cornell, W. D., Cieplak, P. A 2nd generation forcefield for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 117, 5179-5197 (1995).
- Lilien, R. H., Stevens, B. W., Anderson, A. C., Donald, B. R. A novel ensemble-based scoring and search algorithm for protein redesign and its application to modify the substrate specificity of the gramicidin synthetase a phenylalanine adenylation enzyme. J. Comput. Biol. 12, 740-761 (2005).
- Lee, M. R., Baker, D., Kollman, P. A. 2.1 and 1.8 A°Cα RMSD structure predictions on two small proteins, HP-36 and S15. J. Am. Chem. Soc. 123, 1040-1046 (2001).
- Rohl, C. A., Baker, D. De novo determination of protein backbone structure from residual dipolar couplings using rosetta. J. Am. Chem. Soc. 124, 2723-2729 (2002).
- Rohl, C. A., Strauss, C. E. M., Misura, K. M. S., Baker, D. Protein structure prediction using rosetta. Methods Enzymol. 383, 66-93 (2004).
- DiMaggio, P. A., McAllister, S. R., Floudas, C. A., Feng, X. J., Rabinowitz, J. D., Rabitz, H. A. Biclustering via optimal re-ordering of data matrices in systems biology: Rigorous methods and comparative studies. BMC Bioinformatics. 9 (458), (2008).
- DiMaggio, P. A., McAllister, S. R., Floudas, C. A., Feng, X. J., Rabinowitz, J. D., Rabitz, H. A. A network flow model for biclustering via optimal re-ordering of data matrices. J Global Optimization. 47, 343-354 (2010).
- Daily, M. D., Masica, D., Sivasubramanian, A., Somarouthu, S., Gray, J. J. CAPRI rounds 3-5 reveal promising successes and future challenges for RosettaDock. Proteins. 60, 181-186 (2005).
- Gray, J. J., Moughon, S., et al. Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 331, 281-299 (2003).
- Gray, J. J., Moughon, S. E., et al. Protein-protein docking predictions for the CAPRI experiment. Proteins. 52, 118-122 (2003).
- Kuhlman, B., Baker, D. Native protein sequences are close to optimal for their structures. Proc. Natl Acad. Sci. U.S.A. 97, 10383-10388 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved