Method Article
Dynamic Lung Tumor Tracking for Stereotactic Ablative Body Radiation Therapy
In This Article
Summary
Stereotactic ablative body radiation therapy (SBRT) involves the precise delivery of high-dose radiation to cancer tumor targets. A novel SBRT platform offers a first-of-its-kind gimbaled radiation accelerator mounted within a pivoting O-ring gantry capable of dynamic image-guided tumor tracking. Here, we describe dynamic tumor tracking for lung targets.
Abstract
Physicians considering stereotactic ablative body radiation therapy (SBRT) for the treatment of extracranial cancer targets must be aware of the sizeable risks for normal tissue injury and the hazards of physical tumor miss. A first-of-its-kind SBRT platform achieves high-precision ablative radiation treatment through a combination of versatile real-time imaging solutions and sophisticated tumor tracking capabilities. It uses dual-diagnostic kV x-ray units for stereoscopic open-loop feedback of cancer target intrafraction movement occurring as a consequence of respiratory motions and heartbeat. Image-guided feedback drives a gimbaled radiation accelerator (maximum 15 x 15 cm field size) capable of real-time ±4 cm pan-and-tilt action. Robot-driven ±60° pivots of an integrated ±185° rotational gantry allow for coplanar and non-coplanar accelerator beam set-up angles, ultimately permitting unique treatment degrees of freedom. State-of-the-art software aids real-time six dimensional positioning, ensuring irradiation of cancer targets with sub-millimeter accuracy (0.4 mm at isocenter). Use of these features enables treating physicians to steer radiation dose to cancer tumor targets while simultaneously reducing radiation dose to normal tissues. By adding respiration correlated computed tomography (CT) and 2-[18F] fluoro-2-deoxy-ᴅ-glucose (18F-FDG) positron emission tomography (PET) images into the planning system for enhanced tumor target contouring, the likelihood of physical tumor miss becomes substantially less1. In this article, we describe new radiation plans for the treatment of moving lung tumors.
Introduction
Lung cancers account for the greatest number of cancer-related deaths in women and men worldwide2. Up to 63% of persistent or recurrent lung cancers involve lung tissue that is already taxed by chemotherapy or previously irradiated.3,4. Further irradiation at sites of persistent or recurrent lung tumors may lead to intolerable lung morbidity5,6, especially when conventional surgery, chemotherapy, and radiation therapy already have been tried. Thus, women and men in such clinical circumstances have need of new cancer therapy strategies similar to treatments presented before in this journal7. Stereotactic ablative body radiation therapy (SBRT) may satisfy this therapeutic need by sterilizing lung tumors through precisely targeted, high-dose radiation8,9.
There is a novel SBRT platform capable of this therapeutic task10-12. It separates itself from other SBRT platforms by integrating dual-diagnostic Exactrac kV x-ray units (capable of cone-beam computed tomography target localization) and an infrared camera unit (capable of body surface marker tracking as a surrogate for internal motion) that both allow stereoscopic open-loop feedback of cancer target intrafraction motion. It also has a unique ±4 cm gimbaled pan-and-tilt radiation accelerator that has its radiation beam shaped by 60 tungsten alloy leaves (0.25 cm physical width, 11 cm physical height). It uses a full over-center-travel multi-leaf collimator for a maximum 15 x 15 cm field size. It incorporates a robot-driven ±60° pivoting O-ring and ±185° rotational gantry allowing for coplanar and non-coplanar accelerator beam set-up angles and unique treatment degrees of freedom. Lastly, it has submillimeter accuracy (0.4 mm at isocenter)13. In contrast, other SBRT radiotherapy platforms mount a clinical radiation accelerator either to an industrial robotic arm14, or to a helical slice-by-slice gantry15, or within a conventional machine linked to image-guided intensity modulated radiation therapy or dynamic arc delivery software16. Each platform engages a variety of machine components to track motion resulting from respiration motion, heartbeat, or digestion. Lung radiosurgery has had clinical success17,18, rendering the modality a novel treatment option in radiation oncology19,20. This how-to article provides one new radiation therapy protocol that describes dynamic lung tumor tracking for therapeutic treatment intent.
Protocol
Ethical statement: Summa Health System institutional review board approval was obtained for this study.
1. Treatment Consultation
- Describe the new lung SBRT treatment to the patient.
NOTE: The new SBRT platform delivers coplanar and non-coplanar high radiation dose to cancer targets while lowering radiation dose to non-target organs. - Discuss the treatment risks.
NOTE: SBRT may result in possible short-term skin hyperpigmentation or erythema, tiredness, infrequent cough, nausea, esophagitis, and rare visceral organ injury. Pneumonitis, or inflammation of the lung with low-grade fever and non-productive cough, may occur up to three months after treatment. Acute or late injuries to the heart, other muscles, peripheral nerves or spinal cord, and bone are uncommon. There is a very small hazard for radiation-induced malignancy.
2. Fiducial Marker Placement
- Perform percutaneous CT-guided or bronchoscopic placement of a single gold-coated marker inserted into the tumor target center-of-mass.
- Ask a radiologist to perform a 3-5 mm thick contiguous axial tomographic imaging of the patient’s chest7.
- Determine a safe needle approach minimizing aerated lung tissue traversed (avoiding bullae and fissures)7.
- Inject local subcutaneous anesthesia (e.g., 1% lidocaine).
- Introduce 17 or 18 G coaxial needle to place a single short (0.75 x 10 mm) or single long (0.75 x 20 mm) marker10.
- Do an electromagnetic navigation bronchoscopy-guided fiducial marker placement by asking a pulmonologist to acquire tomographic imaging of the chest for endobronchial mapping21.
- Wedge the bronchoscope in the suspected bronchial segment.
- Steer the bronchoscope sensor probe to the target lesion.
- Deploy a fiducial marker by transbronchial needle.
NOTE: Techniques for fiducial-free radiosurgery in the lung are considered investigational and subject to active research22,23.
- As an alternative, order at least three short (1.6 x 3 mm) gold-coated markers to be positioned within a 6 cm ‘box’ around the target. If more than one fiducial marker is placed, a physical separation of 2 cm between markers is recommended.
3. Treatment Planning
- Perform CT-guided simulation (described in steps 3.2 and 3.3) 4-7 days after fiducial marker placement.
- Ask the patient to lie in a supine-head-first on the treatment machine flat tabletop.
- Position the patient’s arms over their head, supported by upper arm and wrist holders or a vacuum-bag immobilizer. Ensure that the thorax and abdomen are not immobilized.
- Optionally, use a two-pin localized knee sponge for indexing.
- Place at least 4 infrared-tracked body markers on the chest for localization. Infrared-tracked breathing markers overly body regions demonstrating consistent vertical respiratory motion (3 mm or more peak-to-peak motion is recommended).
- Conduct a non-contrasted contiguous helical axial CT scan (1 mm slice thickness, voltage 120 kVp, 350 mAs).
NOTE: Treating physicians may order a 4D CT thorax scan12 or a 3D CT thorax scan that includes free-breathing, end-inspiration, and end-expiration breath-hold image sets24. - Obtain 18F-FDG PET/CT scans for enhanced capture of lung tumor motion.
- Ask the patients to lie in a head-first scanning position for contiguous helical CT scan (e.g., voltage 120 kVp, 450 mAs) from the orbitomeatal line to upper thighs during quiet breathing.
- Acquire 18F-FDG PET after intravenous administration of 11 mCi of 18F-FDG on average in the same scanning position from the orbitomeatal line to upper thighs during quiet breathing. If this technique is used, 18F-FDG PET/CT scans are auto-contoured by software set at a 40% SUVmax threshold, and then, co-registered with CT simulation images as described1.
- Contour the primary lung gross target volume or volumes (GTVp) by hand drawing on 4D CT datasets, preferably the exhale phase. Expanding the GTVp with a 5 mm margin creates the planning tumor volume (PTV). Radiation dose planning occurs on the end-expiration phase scan for dynamic tracking.
NOTE: As an alternative and when using 3D CT datasets, the free-breathing CT simulation scan is the reference scan. Using this technique, the treating radiation oncologist contours the GTV on the free-breathing (GTVfb), inspiration (GTVi), and expiration (GTVe) CT simulation scans. A thresholded 40 percent maximum standard uptake value contour on the 18F-FDG PET images creates an 18F-FDG PET clinical target volume (CTVpet)1. A composite ITV represents the added sum of the GTVfb, GTVi, GTVe, and CTVpet volumes. A 5 mm margin expansion of the composite ITV creates a PTV. Here, radiation dose planning occurs on the free-breathing scan for dynamic tracking. - Contour nearby normal tissue structures by hand drawing on 4D CT datasets, preferably the exhale phase. This may include normal lung, heart plus pericardium, esophagus, liver, bilateral kidneys, brachial plexus, and spinal cord. A contour of the trachea, right mainstem bronchus, and left mainstem bronchus may be generated, expanded 3 mm, and used as a high-priority planning constraint to avoid late toxicity airway fibrosis.
- Click on the dynamic tracking button in the planning software. This action engages the gimbaled pan-and-tilt tracking on the new SBRT platform.
- Prescribe a radiation dose to the PTV. Consider one of three radiation Monte Carlo dose prescriptions: 3 x 17 Gy = 51 Gy daily for peripheral lung lesions; 4 x 12 Gy = 48 Gy for central lung lesions and peripheral chest wall lesions; or every other day 5 x 10 Gy = 50 Gy. Uncommonly when PTV volume constraints (i.e., 95% coverage) or organ-at-risk constraints are not met, a prescription of 8 x 7.5 Gy = 60 Gy may be used.
4. Treatment Delivery and Workflow
- Build a quiet breathing correlation model after supine-head-first alignment.
- Place 4 (or up to 6) infrared body markers on the body in the same marked locations identified at CT simulation.
- Verify positional accuracy of the body markers and patient alignment by infrared camera and screens at the treatment console.
NOTE: Body marker position serves as a beam-on check for irregular movement, such a cough. - Acquire cross-plane dual-diagnostic kV x-rays or cone-beam CT images at the treatment console to detect implanted markers for internal positional accuracy.
- Associate and correlate body marker motion (as a surrogate for respiration) and internal implanted marker motion with computer software linked to the new SBRT platform workflow.
NOTE: An alternative localization method involves orthogonal alignment of the patient according to anterior and lateral tattooed CT simulation laser triangulation marks and use of a standardized reference array (star) with six impregnated infrared body markers.
- Generate a lung tumor motion correlation model.
- Derive a gimbal pan-and-tilt path for the accelerator to track tumor motion using computer software linked to the new SBRT platform workflow.
- Visually assess the lung tumor motion correlation model prior to radiation delivery.
- Observe for fiducial marker drift during radiation delivery.
- Evaluate machine-patient collisions due to gantry rotation, O-ring pivot, and gimbal pan-and-tilt actions prior to plan delivery.
NOTE: The radiation staff will perform this step. Treatment may entail five to nine static coplanar and noncoplanar treatment beams, manually and visually checked by radiation delivery staff. Treatments may last 15-30 min, with lung tumor motion correlation model verifications done approximately every 7 min.
Results
SBRT on the new platform currently involves multiple static radiation beams converging on single or multiple closely-associated clinical radiation targets, as depicted for example in Figure 1. A representative good planning outcome delivers ablative radiation with 95% coverage of cancer target volume and cancer target dose conformity. Figure 1 shows 5 coplanar and 4 non-coplanar beams (i.e., ring rotation +20° for beams 2, 4, 6, and 8) used to treat a single PTV representing squamous cell carcinoma in the right lung. Beam margins for the PTV were one-millimeter. Radiation dose, prescribed to the 95% isodose line, rendered 95% PTV coverage with a conformity index of 1.48. The prescription was 50 Gy in five every other day 10 Gy fractions. Structures depicted here include the planning target volume (red), internal target volume (white), spinal cord (green), and esophagus (light blue). Isodose lines are as indicated.
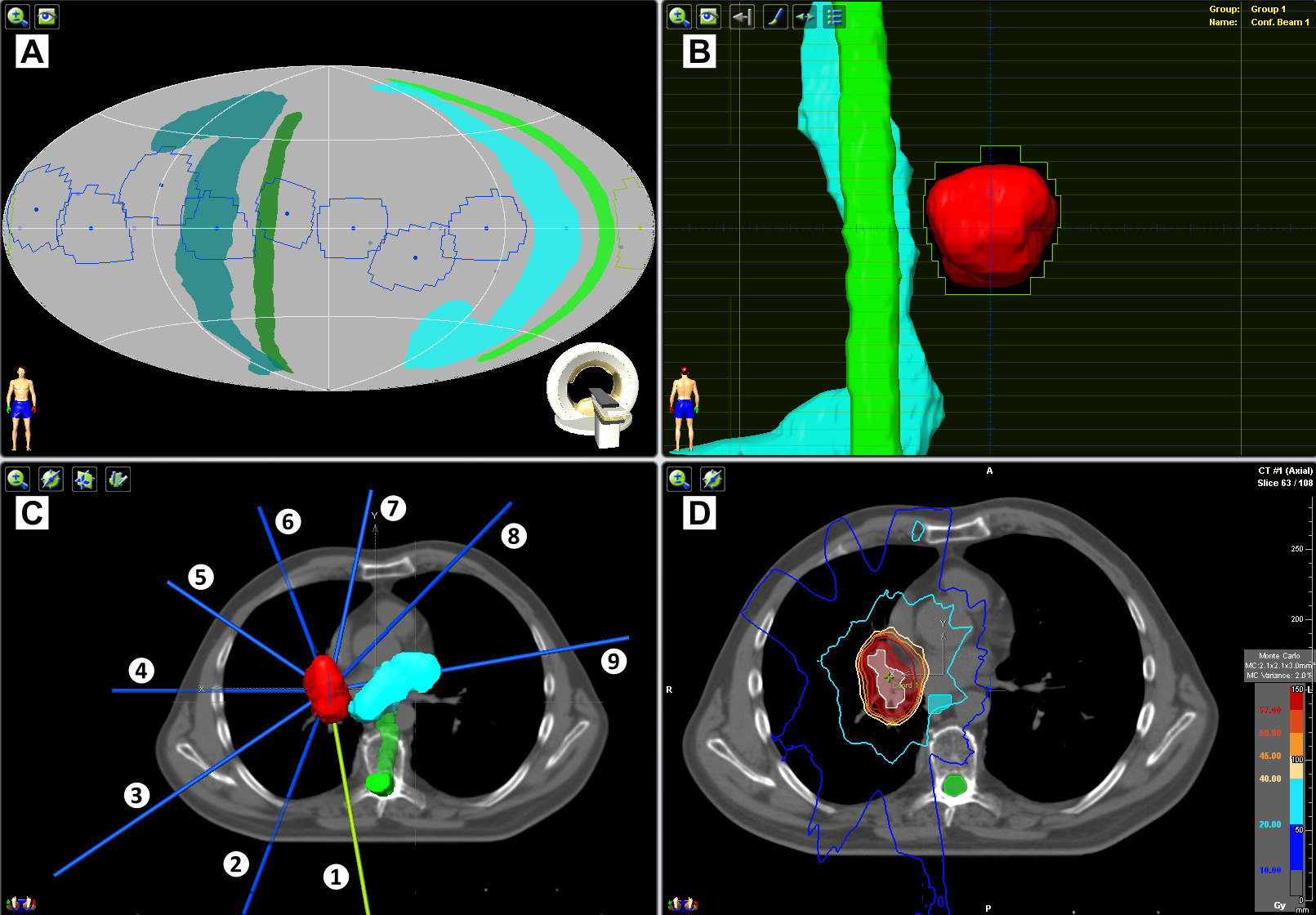
Figure 1: Dynamic tumor tracking of a right-sided lung tumor. Pictured is an example of ablative radiation dose (50 Gy in five 10 Gy every other day fractions) delivered to a single right-sided lung tumor using nine static beams (blue/green, 34° apart). The 4 planning software windows depict: (A) a beam and critical structure collision map, (B) beam’s-eye-view (here, beam 1), (C) three-dimensional CT and beam reconstruction map, and (D) axial CT with dose distribution.
| Structure | Metric | Volume | Acceptable variation |
| PTV | V50Gy | ≥95% | ≥90% |
| Minimum dose | 0.03 cm3 | ≥46 Gy (92%) | ≥45 Gy (90%) |
| Maximum dose | 0.03 cm3 | ≤60 Gy (120%) | ≤62.5 Gy (125%) |
| Spinal cord | 0.03 cm3 | ≤15 Gy | ≤22 Gy |
| Lung (minus GTV) | V20Gy | ≤10% | ≤15% |
| Mean dose | ≤8 Gy | ≤10 Gy | |
| Heart / Pericardium | 15 cm3 | ≤32 Gy | ≤36 Gy |
| Esophagus | Mean dose | ≤18 Gy | ≤20 Gy |
| 0.03 cm3 | ≤27 Gy | ≤30 Gy | |
| Brachial plexus | 0.03 cm3 | ≤24 Gy | ≤30 Gy |
Table 1: Structure treatment planning constraints.
Discussion
Promising early stereotactic radiosurgery clinical experiences have driven clinical trial investigation of ablative radiation for treatment of lung cancers25,26. Experience has led investigators to use ablative radiation against a variety of tumor types metastasizing to the lung27,28. The new SBRT platform introduces a radiation delivery system particularly attuned to the treatment of moving tumors.
The new SBRT platform delivers an unseen x-ray treatment that is generated by a linear accelerator mounted within a pivoting O-ring gantry. A gimbal mechanism enables pan-and-tilt motion of the linear accelerator, providing in-time dynamic tumor motion tracking. Dual cross-plane kV x-rays are obtained before and during treatment to verify 6 degree-of-freedom patient positioning. Coplanar and non-coplanar unique degrees of freedom enhance delivery of high radiation dose to cancer targets while simultaneously minimizing radiation dose to critical visceral organs. It is anticipated that treatment sterilizes cancer cell targets without irreparable damage to normal cells—lowering radiation-related toxicity. Future study of the new SBRT platform will document any gains in target control and any reduction in side effects.
Initial experience with the new SBRT platform shows promise10. Nuances of dynamic tracking of lung tumors continue to be explored; however, some generalizations are apparent. Lung tumors demonstrating motion less than seven millimeters may be best treated by a composite ITV plus 5 mm expansion approach. For lung tumors showing 7 mm or more vertical translation, a dynamic tracking approach using a GTVp plus 5 mm expansion may be best for treatment. Further research defining these limits is needed. Also, 18F-FDG PET images superimposed upon 3D CT image datasets usually increase composite ITV volumes. This approach assumes volume expansion due to 18F-FDG signal smear occurring during the PET scanner’s 3-5 min bin time. A 40% thresholded 18F-FDG clinical target volume has been studied and has been used in one of our programs1. Further research characterizing whether 18F-FDG PET images adequately replicates tumor hysteresis is needed. Lastly, up to 3 lesions in a single lung may be considered for treatment at one time. Otherwise, a sequential approach is done.
Dynamic tracking on the new SBRT platform uses a lung tumor motion correlation model to predict lung tumor motion up to 40 msec into the future. Position and velocity of the infrared body and respiratory markers are included in the model. A marker detection rate of 70% in acquired kV x-rays is a requisite for dynamic tracking. Fiducials are tracked in three dimensions (i.e., x, y, z). Images generated by the kV x-ray units are automatically registered and compared real-time. Observed latency in dynamic tracking is due to limitations in pan-and-tilt gimbal hardware, software processing, and positional control performance of the kV x-ray units. Research investigators are engaged in improving tracking latency.
During radiation delivery using dynamic tracking on the new SBRT platform, it is critical to watch for fiducial marker drift. Trends in fiducial marker drift beyond predefined 3 mm tolerances in any direction results in operator-initiated treatment pause or in automatic beam hold. If a treatment pause occurs, it is recommended that operators allow for resumption of quiet breathing motion over the next several patient breaths and then treatment resumption prior to correlation model rebuild. If pauses are unsuccessful, patient repositioning, infrared breathing marker motion detection, kV marker detection, correlation modeling rebuild are done to resume treatment. In our experience, breathing correlation models are accurate for up to 7 min, often limited by patient tension or relaxation while resting on the treatment tabletop.
Unanswered questions remain. What are the radiobiological consequences and mode of cell death in normal cells and cancer cells occurring after ablative radiation dose? Why has it been so difficult to merge high-precision ablative radiation with radiosensitizing chemotherapies? While it is essential to investigate other modalities of delivering ablative radiation in the chest, it remains unclear as to whether ablative radiation can provide equivalent therapeutic effectiveness as thoracic surgery. Indeed, thoracic surgery is the more commonly used and validated technique to achieve tumor eradication in the lung when conventional therapies have already been applied. Here, the new SBRT platform provides an innovative non-invasive means of therapy for women and men with lung tumors showing motion.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Summa Cancer Institute.
Materials
| Name | Company | Catalog Number | Comments |
| Vero SBRT Linac System 1.0 | Brainlab, Inc. (Munich, Germany) Mitsubishi Heavy Industries, Ltd. (Tokyo, Japan) | 46300 | High accuracy first-of-its-kind gimbaled irradiation head with tilt function and gantry rotation |
| Visicoil fiducial marker | IBA Dosimetry America (Bartlett, TN, USA) | 67245 | 0.75 x 10 mm marker or 0.75 x 20 mm marker |
| Gold fiducial marker | Civco Medical Solutions (Orange City, IA, USA) | MTNW887860 | Sterile placement needle (14 G ETW x 20 cm) with one 1.6 x 3 mm marker |
References
- Kunos, C., et al. 18FDG-PET/CT definition of clinical target volume for robotic stereotactic body radiosurgery treatment of metastatic gynecologic malignancies. J Nucl Med Radiat Ther. S4:001, (2011).
- Ferlay, J., et al. . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. , (2013).
- Albain, K., et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 374, 379-386 (2009).
- Herbst, R. S., et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 23, 5892-5899 (2005).
- Trovo, M., et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 88, 1114-1119 (2014).
- Kelly, P., et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys. 78, 1387-1393 (2010).
- Kunos, C., Brindle, J., DeBernardo, R. Stereotactic radiosurgery for gynecologic cancer. J Vis Exp. 62, e3793 (2012).
- Bral, S., et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys. 80, 1343-1349 (2011).
- Engels, B., et al. Phase II study of helical tomotherapy in the multidisciplinary treatment of oligometastatic colorectal cancer. Radiat Oncol. 7, 34 (2012).
- Depuydt, T., et al. Treating patients with real-time tumor tracking using the Vero gimbaled linac system: Implementation and first review. Radiother Oncol. , (2014).
- Poels, K., et al. A complementary dual-modality verification for tumor tracking on a gimbaled linac system. Radiother Oncol. 109, 469-474 (2013).
- Depuydt, T., et al. Initial assessment of tumor tracking with a gimbaled linac system in clinical circumstances: a patient simulation study. Radiother Oncol. 106, 236-240 (2013).
- Depuydt, T., et al. Computer-aided analysis of star shot films for high-accuracy radiation therapy treatment units. Phys Med Biol. 57, 2997-3011 (2012).
- Adler, J. J., et al. The CyberKnife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 69, 124-128 (1997).
- Mackie, T., et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Medical Physics. 20, 1709-1719 (1993).
- Benedict, S., et al. Intensity-modulated stereotactic radiosurgery using dynamic micro-multileaf collimation. Int J Radiat Oncol Biol Phys. 50, 751-758 (2001).
- Zheng, X., et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 90, 603-611 (2014).
- Widder, J., et al. Pulmonary oligometastases: metastasectomy or stereotactic ablative radiotherapy. Radiother Oncol. 107, 409-413 (2013).
- Mitera, G., et al. Cost-effectiveness analysis comparing conventional versus stereotactic body radiotherapy for surgically ineligible stage I non-small-cell lung cancer. Journal of oncology practice / American Society of Clinical Oncology. 10, e130-e136 (2014).
- Bijlani, A., Aguzzi, G., Schaal, D. W., Romanelli, P. Stereotactic radiosurgery and stereotactic body radiation therapy cost-effectiveness results. Front Oncol. 3, 77 (2013).
- Harley, D. P., et al. Fiducial marker placement using endobronchial ultrasound and navigational bronchoscopy for stereotactic radiosurgery: an alternative strategy. The Annals of thoracic surgery. 89, 368-373 (2010).
- Bibault, J. E., et al. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol. 7, 102 (2012).
- Bahig, H., et al. Predictive parameters of CyberKnife fiducial-less (XSight Lung) applicability for treatment of early non-small cell lung cancer: a single-center experience. Int J Radiat Oncol Biol Phys. 87, 583-589 (2013).
- Kunos, C. Image-guided motion management. OMICS J Radiology. 2, e120 (2013).
- Fakiris, A. J., et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 75, 677-682 (2009).
- Chang, J. Y., et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 72, 967-971 (2008).
- Kunos, C., et al. Phase II clinical trial of robotic stereotactic body radiosurgery for metastatic gynecologic malignancies. Front Oncol. 2, 181 (2012).
- Ricardi, U., et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer. 75, 77-81 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved