A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
High Speed Sub-GHz Spectrometer for Brillouin Scattering Analysis
In This Article
Summary
Here we present a protocol to build a rapid Brillouin spectrometer. Cascading virtually imaged phase array (VIPA) etalons achieve a measurement speed more than 1,000 times faster than traditional scanning Fabry-Perot spectrometers. This improvement provides the means for Brillouin analysis of tissue and biomaterials at low power levels in vivo.
Abstract
The goal of this protocol is to build a parallel high-extinction and high-resolution optical Brillouin spectrometer. Brillouin spectroscopy is a non-contact measurement method that can be used to obtain direct readouts of viscoelastic material properties. It has been a useful tool in material characterization, structural monitoring and environmental sensing. In the past, Brillouin spectroscopy has usually employed scanning Fabry-Perot etalons to perform spectral analysis. This process requires high illumination power and long acquisition times, making the technique unsuitable for biomedical applications. A recently introduced novel spectrometer overcomes this challenge by employing two VIPAs in a cross-axis configuration. This innovation enables sub-Gigahertz (GHz) resolution spectral analysis with sub-second acquisition time and illumination power within the safety limits of biological tissue. The multiple new applications facilitated by this improvement are currently being explored in biological research and clinical application.
Introduction
Brillouin scattering, first described by Leon Brillouin1 in 1922, is the inelastic scattering of light from the thermal acoustic modes in a solid and from the thermal density fluctuations in a liquid or gas. The spectral shift of the scattered light, usually in the sub GHz-range, provides information about the interaction between the incident light and the acoustic phonons in the sample. As a result, it can provide useful information regarding the viscoelastic properties of the examined material.
In its spontaneous version, Brillouin scattering generally has cross-sections in the order of Raman scattering, resulting in a very weak signal. Additionally, Brillouin frequency shifts are orders of magnitude smaller than Raman shifts. As a consequence, elastically scattered light (from Rayleigh or Mie scattering), stray light, and back-reflections off of the sample can all easily overshadow the Brillouin spectral signature. Hence, a Brillouin spectrometer needs to not only achieve sub-GHz spectral resolution but also high spectral contrast or extinction.
In traditional Brillouin spectrometers these requirements are met by scanning-grating monochromators, optical beating methods, and, most popularly, multiple-pass scanning Fabry-Perot interferometers2. These methods measure each spectral component sequentially. This approach leads to acquisition times for a single Brillouin spectrum ranging from a few minutes to several hours, depending on the instrument and on the sample. The two-stage VIPA spectrometer, built using this protocol, has the ability to collect all of the spectral components within less than a second while providing sufficient extinction (>60 dB) to effectively suppress other spurious signals2.
The integration of the VIPA etalons is the key element of this spectrometer. A VIPA is a solid etalon with three different coating areas: in the front surface, a narrow anti-reflection coating strip allows the light to enter the VIPA, while the rest of the surface features a highly reflective (HR) coating; in the back surface, a partially reflective coating allows a small portion (~5%) of the light to be transmitted. When focused onto the narrow entrance of the slightly tilted VIPA, the light beam gets reflected into sub-components with fixed phase difference within the VIPA2. Interference between the sub components achieves the aspired high spectral dispersion. Aligning two VIPAs sequentially in cross-axis configuration introduces spectral dispersion in orthogonal directions3. The spectral dispersion in orthogonal directions spatially separates the Brillouin peaks from unwanted crosstalk, which makes it possible to pick up only the Brillouin signal. Figure 1 displays a schematic of the two stage VIPA spectrometer. The arrows below the optical elements indicate the degree of freedom in which the translational stages should be oriented.

Figure 1. Instrumental setup. An optical fiber delivers the Brillouin scattering into the spectrometer. A cylindrical lens C1 (f=200 mm) focuses the light into the entrance of the first VIPA (VIPA1). Another cylindrical lens C2 (f=200 mm) maps the spectral angular dispersion into a spatial separation in the focal plane of C2. In this plane, a vertical mask is used to select the desired portion of the spectrum. An analogous configuration follows, tilted at 90 degrees. The beam passes through a spherical lens S1 (f=200 mm) and is focused into the entrance slit of the second VIPA (VIPA2). A spherical lens S2 (f=200 mm) creates the two-dimensional spectrally separated pattern in its focal plane, where another horizontal mask is placed. The horizontal mask is imaged onto the EMCCD camera using an achromatic lens pair. Please click here to view a larger version of this figure.
An undergraduate student with some optics coursework and basic aligning experience should be able to build and use this two-stage spectrometer. The spectrometer has recently been shown to be compatible with a variety of standard optical probes3,4,5 (e.g., confocal microscope, endoscope, slit-lamp ophthalmoscope). Here, the spectrometer is connected to a confocal microscope. The laser light is aligned into a standard research inverted system microscope after integrating a 90:10 beam splitter. The backscattering light from the sample is coupled into a single mode fiber, making the microscope confocal.
Protocol
Note: Brillouin spectral analysis requires a single-longitudinal mode laser (~10 mW at the sample). For aligning purposes, use a strongly attenuated portion of this laser beam (<0.1 mW).
1. Initial Setup of Fiber and the EMCCD (Electron Multiplied Charge Coupled Device) Camera
- Identify about 1,600 mm free aligning space for the spectrometer on an optical table.
- Mount the EMCCD camera at the end of the free aligning space.
- Use set screws to attach the camera to posts. Tighten the posts in post holders at the desired optical axis height. Tighten the post holders onto the optical table using table clamps.
- Turn on the EMCCD camera. Be mindful to avoid camera saturation.
- Install camera software on lab computer and connect the camera to the computer.
- Disable the gain and set low integration time (~0.01 sec). Start acquiring EMCCD images. Observe the camera images on the computer screen.
- Mount the fiber collimator about 1,600 mm in front of the camera.
- Place the fiber collimator in the fiber collimator adapter.
- Mount the adapter onto a post. Tighten the post into a post holder at the approximate height of the optical axis (height of the camera). Tighten post holder onto optical table using table clamps.
- Align the output beam along the optical axis.
- Use a set screw to connect a standard iris to a post. Tighten the post into a post holder.
- Place the iris in front of the fiber collimator (<50 mm). Adjust the height of the iris to the beam. Move the iris along the desired optical axis, which should point directly into the camera.
- Insert an optical density filter directly after the laser output if the camera saturates. Use the adjustment screws of the fiber collimator mount to align the beam along the optical axis. Once this is achieved, the beam will cleanly pass through the iris along the entire beam path.
- Mount a matched achromatic lens pair (f=30 mm) in front of the camera.
2. Horizontal Stage of Spectrometer
- Mount the horizontal mask.
- Use set screws to attach the horizontal mask onto a post. Tighten the post into a post holder. Mount the post holder onto a translational stage, allowing the mask to slide horizontally in and out of the beam path. (Figure 1)
- Use cap screws to tighten the translational stage on the optical table such that the mask is imaged sharply onto the CCD camera at the focal distance (f=30 mm) of the achromatic lens pair.
- Mount and align the spherical lens S1 (f=200 mm) 600 mm in front of the horizontal mask.
- Use set screws to mount S1 onto a post holder. Tighten the post into a post holder.
- Mount two irises as described in 1.5.1. Before placing S1 in the beam path, insert one iris before and one iris after the desired S1 position (600 mm in front of the horizontal mask). Adjust the height of the irises such that the beam cleanly passes through both of them.
- Place S1 600 mm in front of the horizontal mask (Figure 1). Adjust height and angle of S1 until both, the back reflection off of the lens and the out-coming beam, cleanly pass through the irises. Tighten the post holder holding S1 onto the optical table using table clamps.
- Mount VIPA2 in the focal plane of the spherical lens (200 mm in front of S1).
- Mount the VIPA holder onto a post using the screws provided with the holder. Tighten the post into a post holder. Tighten the post holder onto a horizontal translation stage. (Figure 1)
- Place VIPA2 carefully in the VIPA holder with the entrance slit oriented vertically. (Figure 1)
- Fine-adjust the position of the VIPA mount to be exactly in the focal plane of S1. (Check by tracing the beam waist with a white card.)
- Use cap screws to tighten the translational stage onto the optical table and slide VIPA2 out of the beam using the degree of freedom of the translational stage.
- Mount and align the spherical lens S2 (f=200 mm) 200 mm after the VIPA and 200 mm in front of the horizontal mask. Follow the procedure described in 2.2.1-2.2.3. Instead of attaching the post holder onto the optical table, mount it onto a translational stage with its degree of freedom oriented along the optical axis. (Figure 1) Use cap screws to tighten the translational stage onto the optical table.
- Use the horizontal translational stage (2.3.4) to slide the VIPA2 entrance into the beam path. Observe vertical lines on the camera image.
- Fine-adjust S2 using the translational stage mounted in 2.4 until the vertical lines on the camera image appear sharp.
- Adjust the spectrum with the horizontal-tilt degree of freedom on the VIPA holder and the translational stage. Use the horizontal-translation degree of freedom to tune the entrance position of the beam into the etalon. Use the horizontal-tilt degree of freedom to tune the input angle of the beam into the etalon.
- Measure finesse and throughput.
- Take an image by pressing F5 or alternatively by clicking “acquisition setup” in the upper left hand corner and choosing the option “take image” in the camera software.
- Right click on the image and choose the option “line plot”. Drag the cursor horizontally across the image to generate a line plot. Release the cursor to observe the generated line plot.
- Use the line plot to measure the finesse. Divide the distance between two peaks by their full width at half maximum (FWHM). Aim for >30.
- Determine the throughput by measuring the power with a power meter immediately before and after VIPA2. Aim for >50%.
- Tune the input angle of the beam into the etalon with the degree of freedom on the VIPA holder (2.6) and observe the trade-off between finesse and throughput.
- If finesse and throughput are not satisfactory go back and fine-adjust the alignment. Make sure VIPA2 is in the focal plane of S1. Repeat steps 2.3.3 -2.7.
3. Vertical Stage of Spectrometer
- Slide the VIPA (VIPA2), aligned in part 2, out of the beam using the translational stage (2.3.4). The horizontal stage of the spectrometer will now behave as a 1:1 imaging system.
- Mount the vertical mask.
- Use set screws to attach the vertical mask onto a post. Tighten a post into a right angle post clamp adapter. Tighten a second post into the right angle adapter and put it into a post holder. Mount the post holder onto a vertical translational stage, allowing the mask to slide vertically in and out of the beam path. (Figure 1)
- Use cap screws to tighten the vertical translational stage onto the optical table such that the vertical mask is imaged sharply onto the EMCCD camera 200 mm in front of S1.
- Mount and align the cylindrical lens C1 (f=200 mm) 600 mm in front of the vertical mask.
- Screw the cylindrical lens holder onto a post. Tighten the post into a post holder. Carefully place C1 into the lens holder and tighten the screws to fix it in place.
- Mount two irises as described in 1.5.1. Before placing C1 in the beam path, insert one iris before and one iris after the desired S1 position (600 mm in front of the vertical mask). Adjust the height of the irises such that the beam cleanly passes through both of them.
- Place C1 600 mm in front of the vertical mask (Figure 1). Carefully adjust the height, tilt, and lateral position of C1 until both, the back reflection off of the lens and the out-coming beam, are centered onto the irises. Tighten the post holder holding C1 onto the optical table using table clamps.
- Mount VIPA1 in the focal plane of the cylindrical lens (200 mm in front of C1).
- Mount the VIPA holder onto a post using the screw provided with the holder. Tighten the post into a post holder. Tighten the post holder onto a vertical translation stage. (Figure 1)
- Place VIPA1 carefully into the VIPA holder with the entrance slit oriented horizontally. (Figure 1)
- Fine-adjust the position of the VIPA mount to place VIPA1 exactly in the focal plane of C1. (Check by tracing the beam waist with a white card.)
- Use cap screws to tighten the vertical translational stage onto the optical table and slide VIPA1 out of the beam using the degree of freedom of the translational stage.
- Mount and align the cylindrical lens C2 (f=200 mm) 200 mm after VIPA1 and 200 mm in front of the vertical mask, following the procedure described in 3.3.1-3.3.3. Instead of attaching the post holder onto the optical table, mount it onto a translational stage with its degree of freedom oriented along the optical axis. (Figure 1) Use cap screws to tighten the translational stage onto the optical table.
- Use the translation stage (3.4.4) to slide the VIPA1 entrance into the beam path. Observe horizontal lines on the camera image.
- Fine-adjust C2 using the translational stage mounted in 3.5 until the horizontal lines on the camera image appear sharp.
- Adjust the spectrum with the vertical-tilt degree of freedom on the VIPA holder and the translational stage. Use the vertical-translation degree of freedom to tune the entrance position of the beam into the etalon. Use the vertical-tilt degree of freedom to tune the input angle of the beam into the etalon.
- Measure finesse and throughput.
- Follow steps 2.7.1 -2.7.2 to generate a line plot vertically across an image of the camera screen.
- Use the line plot to measure the finesse by dividing the distance between two horizontal lines by their full width at half maximum (FWHM). Aim for > 40.
- Measure the throughput by measuring the power with a power meter immediately before and after VIPA1. Aim for >30%.
- Tune the vertical input angle of the beam into the etalon with the vertical-tilt degree of freedom on the VIPA holder (3.7). Observe the trade-off between finesse and throughput.
- If finesse and throughput are not satisfactory go back and fine-adjust the alignment. Make sure VIPA1 is in the focal plane of C1. Repeat step 3.4.3-3.8.
4. Combination of the Two Stages and Final Alignment
- Slide in VIPA2. Observe horizontally and vertically spaced dots. These dots are the spectral signature of the single frequency laser. Adjust the translational stages of the VIPAs until the dots are in sharp focus.
- Measure finesse and throughput of the spectrometer.
- Follow steps 2.7.1.-2.7.2 to generate a line plot diagonally across two of the laser dots.
- Use the line plot to measure finesse by dividing the diagonal distance between two dots by their full width at half maximum (FWHM). Aim for >30.
- Build a black box to enclose the spectrometer.
- Use construction rails to build the box skeleton, which should enclose the entire spectrometer from the fiber collimator to the CCD camera (63 in x 9 in x 15 in).
- Cover the box skeleton with Blackout Fabric and tape it tight at the corners. Ensure that the masks and the VIPAs are easily accessible.
- Connect the fiber to a standard optical probe, such as a reflectance confocal microscope4. Standard optical probes used to collect back-scattered light will carry a Brillouin signal.
5. Measuring the Brillouin Shift
- Close both the vertical and the horizontal masks until the laser signature disappears. Move the translation stages accordingly.
- Enable the gain and increase the integration time of the camera as much as possible without saturating the EMCCD camera.
- Observe the Brillouin shift of a sample.
- Place a sample in the focus of a confocal microscope (or other optical probe). The spatial resolution will depend on the objective lens used in the confocal microscope. Use a plastic dish or a cuvette for liquids. Use Methanol for the first measurement.
- To optimize the spectrometer throughput, open one mask at a time and scan through the spectrometer orders by tilting the VIPA and adjusting its translation stage. Find the order in which the signal appears the strongest. Close mask again until the laser signal disappears. Repeat with the other mask and VIPA (described in 2.6 and 3.7).
- Take a measurement of the sample.
- Take an image of the spectrum following step 2.7.1.
- Obtain calibration measurements by taking an image of the spectrum of water and glass (or other sample with known Brillouin shift). Save the image by clicking “file” in the upper left hand corner and choosing the option “save as”. Save the image in “.sif” format if the data analysis is performed in the camera software. Save the image in “.tif” format if the data analysis is performed in another computational software program.
6. Calibration and Analysis of Brillouin Spectrum
- Determine the free spectral range (FSR) and the EMCCD pixel to optical frequency conversion ratio (PR) in the Brillouin spectrometer.
- Load the data in the camera software or another computational software program.
- Follow step 2.7.2 to generate a line plot across the spectrum of the water calibration image.
- Fit the two peaks of the spectrum with Lorentzian curves to determine peak positions in terms of pixel position (PWater-S, PWater-AS). Alternatively, read out the peak positions manually by taking the highest point of the peaks.
- Repeat step 6.1.2-6.1.3 with the spectrum of the glass calibration image.
- Alternatively to 6.1.1-6.1.4, add both EMCCD frames and fit all four peaks at once. (Figure 2)
- Calculate the PR using equation 1. Plug in the values for PWater-AS and PGlass-AS determined in 6.1.3 and 6.1.4. ΩGlass-AS is known to be 29.3 GHz. In this case, since the free spectral range is only 20 GHz it will appear aliased with the frequency shift of 9.3 GHz. For simplicity use 9.3 GHz for ΩGlass-AS. Use 7.46 GHz for ΩWater-AS.

- Calculate the FSR using equation 2. Plug in the values for PGlass-S, PGlass-AS and PR, calculated in 6.16. Use 9.3 GHz for ΩGlass-AS.

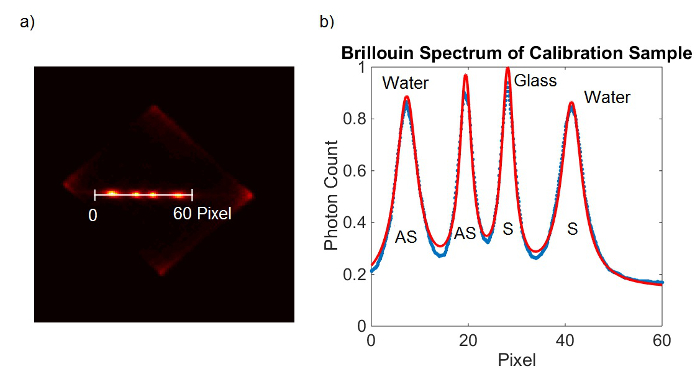
Figure 2. Spectrometer calibration. (A) EMCCD camera frame obtained from calibration sample. (B) Lorentzian curve fit (red) to the measured data (blue). Please click here to view a larger version of this figure.
- Determine the Brillouin shift of a sample.
- Follow step 2.7.2 to generate a line scan across the spectrum of the sample.
- Fit the two peaks of the spectrum with Lorentzian curves to determine peak positions in terms of pixel position. Alternatively, read out the peak positions manually by taking the highest point of the peaks.
- Use the following equations 4 and 5 and the previously calculated values for FSR and PR to calculate the Brillouin shift of the sample.
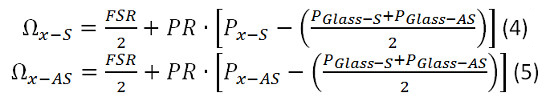
Results
Figure 3 shows representative Brillouin spectra and their fits for different materials. The VIPAs both have a thickness of 5 mm which results in a FSR of approximately 20 GHz. The integration time for these measurements was 100 msec. 100 measurements were taken and averaged. One calibration measurement was taken prior to acquiring the spectra.
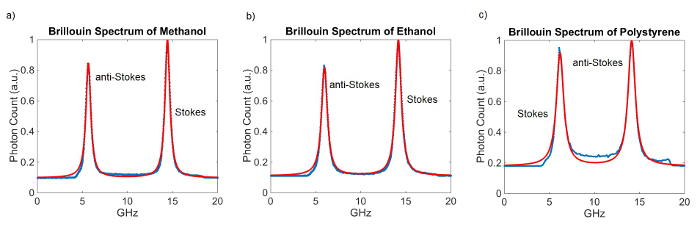
Figure 3. Brillo...
Discussion
A key design feature of this spectrometer configuration is that the two stages can be aligned independently. When a VIPA etalon is slid out of the optical path, the remaining lenses of the spectrometer stage form a 1:1 imaging system, so that the spectral pattern from each stage is imaged onto the CCD camera. Therefore, it is straightforward to go back to either one of the stages to improve its performance without affecting the alignment of the other stage. The set of translational stages and degrees of freedom suggested...
Disclosures
Giuliano Scarcelli and Seok H. Yun hold patents related to Brillouin spectroscopy technology.
Acknowledgements
This work was supported in part by the National Institutes of Health (P41-EB015903, R21EY023043, K25EB015885), National Science of Foundation (CBET-0853773) and Human Frontier Science Program (Young Investigator Grant).
Materials
| Name | Company | Catalog Number | Comments |
| OPTICS | |||
| VIPA (virtual image phase array) | LIGH MACHINERY | Quantity: 2 | |
| Bundle of Three 423 Linear Stages with SM-25 Micrometers | NEWPORT | 423-MIC | Quantity: 1 |
| SS Crossed-Roller Bearing Translation Stage, 0.5 in., 8-32, 1/4-20 | NEWPORT | 9066-X | Quantity: 1 |
| Vernier Micrometer, 13 mm Travel, 9 lb Load Capacity, 50.8 TPI | NEWPORT | SM-13 | Quantity: 1 |
| Adjustable Width Slit | NEWPORT | SV-0.5 | Quantity: 2 |
| Compact Dovetail Linear Stage, 0.20 in. Z Travel, 1.57x1.57x1.38 in. | NEWPORT | DS40-Z | Quantity: 2 |
| Slotted Base Plate, 25 or 40 mm to 65 mm Stage, 1.1 in. Range | NEWPORT | B-2B | Quantity: 2 |
| Ø1/2" Optical Post, 8-32 Setscrew, 1/4"-20 Tap, L = 2", 5 Pack | THORLABS | TR2-P5 | Quantity: 2 |
| Ø1/2" Post Holders, Spring-Loaded Hex-Locking Thumbscrews, L = 2", 5 Pack | THORLABS | PH2-P5 | Quantity: 1 |
| Ø1/2" Post Holders, Spring-Loaded Hex-Locking Thumbscrew, L = 3", 5 Pack | THORLABS | PH3-P5 | Quantity: 1 |
| Imperial Lens Mount For 2" Optics, 8-32 Tap | THORLABS | LMR2 | Quantity: 2 |
| f=200.0 mm, Ø2" Achromatic Doublet, ARC: 400-700 nm | THORLABS | AC254-200-A | Quantity: 2 |
| Kinematic Mount for up to 1.3" (33 mm) Tall Rectangular Optics, Right Handed | THORLABS | KM100C | Quantity: 2 |
| Fixed Cylindrical Lens Mount, Max Optic Height: 1.60" (40.6 mm) | THORLABS | CH1A | Quantity: 2 |
| f=200.00 mm, H=30.00 mm, L=32.0 mm, N-BK7 Plano-Convex Cylindrical Lens, Antireflection Coating: 350-700 nm | THORLABS | L1653L1-A | Quantity: 2 |
| Right-Angle Post Clamp, Fixed 90° Adapter | THORLABS | RA90 | Quantity: 1 |
| Adapter with External C-Mount Threads and Internal SM1 Threads | THORLABS | SM1A9 | Quantity: 1 |
| Studded Pedestal Base Adapter, 1/4"-20 Thread | THORLABS | PB4 | Quantity: 2 |
| Spacer, 2" x 3", 1.000" Thick | THORLABS | Ba2S7 | Quantity: 2 |
| 543 nm, f=15.01 mm, NA=0.17 FC/APC Fiber Collimation Pkg. | THORLABS | F260APC-A | Quantity: 1 |
| SM1-Threaded Adapter for Ø11 mm collimators | THORLABS | Ad11F | Quantity: 1 |
| Translating Lens Mount for Ø1" Optics, 1 Retaining Ring Included | THORLABS | LM1XY | Quantity: 1 |
| Single Mode Patch Cable, 450 - 600 nm, FC/APC, 2 m Long | THORLABS | P3-460B-FC-2 | Quantity: 1 |
| 1:1 Matched Achr. Pair, f1=30 mm, f2=30 mm, BBAR 400-700 nm | THORLABS | MAP103030-A | Quantity: 1 |
| SM1 Lens Tube…length to adjust depend on CCD, we have 3.5 inches | THORLABS | SM1LXX | Quantity: 1 |
| Base Adapters for Ø1/2" Post Holders and Ø1" Posts | THORLABS | BE1 | Quantity: 8 |
| Clamping Forks for Ø1/2" Post Holders and Ø1" Posts | THORLABS | CF125 | Quantity: 8 |
| HW-KIT5 - 4-40 Cap Screw and Hardware Kit for Mini-Series | THORLABS | HW-KIT5 | Quantity: 1 |
| D20S - Standard Iris, Ø20.0 mm Max Aperture | THORLABS | D20S | Quantity: 2 |
| FOR ENCLOSURE | |||
| 25 mm Construction Rail, L = 21" | THORLABS | XE25L21 | Quantity: 6 |
| 1" Construction Cube with Three 1/4" (M6) Counterbored Holes | THORLABS | RM1G | Quantity: 8 |
| Right-Angle Bracket for 25 mm Rails | THORLABS | XE25A90 | Quantity: 12 |
| 25 mm Construction Rail, L = 15" | THORLABS | XE25L15 | Quantity: 4 |
| 25 mm Construction Rail, L = 9" | THORLABS | XE25L09 | Quantity: 8 |
| High Performance Black Masking Tape, 2" x 60 yds. (50 mm x 55 m) Roll | THORLABS | T743-2.0 | Quantity: 1 |
| Low-Profile T-Nut, 1/4"-20 Tapped Hole, Qty: 10 | THORLABS | XE25T3 | Quantity: 1 |
| 1/4"-20 Low-Profile Channel Screws (100 Screws/Box) | THORLABS | SH25LP38 | Quantity: 1 |
| 60" (W) x 3 yds. (L) x 0.005" (T) (1.5 m x 2.7 m x 0.12 mm) Blackout Fabric | THORLABS | BK5 | Quantity: 1 |
| CAMERA, LASER and MICROSCOPE | |||
| EMCCD camera | ANDOR | iXon Ultra 897 | Quantity: 1 |
| 400 mW single mode green laser | LASER QUANTUM | torus 532 | Quantity: 1 |
| Research Inverted System Microscope | OLYMPUS | IX71 | Quantity: 1 |
References
- Brillouin, L. Diffusion de la lumiere et des rayonnes X par un corps transparent homogene; influence del'agitation thermique. Ann. Phys. (Paris) . 17, 88-122 (1922).
- Scarcelli, G., Yun, S. H. Multistage VIPA etalons for high-extinction parallel Brillouin spectroscopy. Opt. Exp. 19 (11), 10913-10922 (2011).
- Scarcelli, G. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat. Phot. 2 (1), 39-43 (2008).
- Nichols, A. J., Evans, C. L. Video-rate Scanning Confocal Microscopy and Microendoscopy. J. Vis. Exp. (56), e3252 (2011).
- Steelman, Z., Meng, Z., Traverso, A. J., Yakovlev, V. V. Brillouin spectroscopy as a new method of screening for increased CSF total protein during bacterial meningitis. J. Biophoton. 8 (5), 1-7 (2014).
- Koski, K. J., Yarger, J. L. Brillouin imaging. Appl. Phys. Lett. 87 (6), 061903 (2005).
- Faris, G. W., Jusinski, L. E., Hickman, A. P. High-resolution stimulated Brillouin gain spectroscopy in glasses and crystals. J. Opt. Soc. Am. B. 10 (4), 587-599 (1993).
- Scarcelli, G., Yun, S. H. In vivo Brillouin optical microscopy of the human eye. Opt. Exp. 20 (8), 9197-9202 (2012).
- Scarcelli, G., Besner, S., Pineda, R., Kalout, P., Yun, S. H. In Vivo Biomechanical Mapping of Normal and Keratoconus Corneas. Jama Ophtalmol. , (2015).
- Scarcelli, G., Kim, P., Yun, S. H. In Vivo Measurement of Age-Related Stiffening in the Crystalline Lens by Brillouin Optical Microscopy. Biophys. J. 101 (6), 1539-1545 (2011).
- Antonacci, G., Foreman, M. R., Paterson, C., Török, P. Spectral broadening in Brillouin imaging. Appl. Phys. Lett. 103 (22), 221105 (2013).
- Meng, Z., Traverso, A. J., Yakovlev, V. Background clean-up in Brillouin microspectroscopy of scattering medium. Opt. Exp. 22 (5), 5410-5415 (2014).
- Reiss, S., Burau, G., Stachs, O., Guthoff, R., Stolz, H. Spatially resolved Brillouin spectroscopy to determine the rheological properties of the eye lens. Biomed. Opt. Exp. 2 (8), 2144-2159 (2011).
- Scarcelli, G., Kling, S., Quijano, E., Pineda, R., Marcos, S., Yun, S. H. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest. Ophthalmol. Vis. Sci. 54 (2), 1418-1425 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved