A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Three-Dimensional Culture Assay to Explore Cancer Cell Invasiveness and Satellite Tumor Formation
In This Article
Summary
Cancer cells are embedded in a collagen gel and then sandwiched in an acellular fibrin gel to generate a 3D culture system in which the invasiveness and formation of satellite tumors may be monitored.
Abstract
Mammalian cell culture in monolayers is widely used to study various physiological and molecular processes. However, this approach to study growing cells often generates unwanted artifacts. Therefore, cell culture in a three-dimensional (3D) environment, often using extracellular matrix components, emerged as an interesting alternative due to its close similarity to the native in vivo tissue or organ. We developed a 3D cell culture system using two compartments, namely (i) a central compartment containing cancer cells embedded in a collagen gel acting as a pseudo-primary macrospherical tumor and (ii) a peripheral cell-free compartment made of a fibrin gel, i.e. an extracellular matrix component different from that used in the center, in which cancer cells can migrate (invasion front) and/or form microspherical tumors representing secondary or satellite tumors. The formation of satellite tumors in the peripheral compartment is remarkably correlated to the known aggressiveness or metastatic origin of the native tumor cells, which makes this 3D culture system unique. This cell culture approach might be considered to assess cancer cell invasiveness and motility, cell-extracellular matrix interactions and as a method to evaluate anti-cancer drug properties.
Introduction
Investigating the fundamental and biomedical characteristics of cancer cell invasion/migration and subsequent metastasis establishment is the subject of an intense research1,2. Metastasis is the ultimate stage of cancer and its clinical management remains elusive. A better understanding of metastasis at the cellular and molecular levels will enable the development of more efficient therapies3.
Several properties of metastatic cells can be explored in vitro4 including their stemness and potential to acquire a transition state (e.g., epithelioid-mesenchymal transition) to migrate and invade within and from the primary tumor5. However, the in vitro assessment of invasion/metastasis processes has been a challenge since it virtually excludes the contribution of the blood/lymphatic circulation. Organotypic cultures that embed tumor fragments in collagen gels have previously been used to monitor cancer aggressiveness. Although the complexity of tumors is preserved (e.g., the presence of non-cancerous cells), tumor fragments are exposed to limited medium diffusion, to sampling variation, and to an overgrowth of stromal cells6. An alternative method consists in growing cancer cells within components of the extracellular matrix (ECM), which mimics the three-dimensional (3D) cell environment. The proliferation of breast cancer cell lines in a collagen gel and/or a basement membrane-derived matrix is amongst the best-characterized examples of 3D cell culture. By using specific 3D cell culture environments, the disorganized assembly observed for breast cancer cells grown under standard conditions can be reversed to the spontaneous formation of mammary acini and tubular structures7-10. Furthermore, the formation of multicellular tumor spheroids derived from adenocarcinoma cancer cells congregated using different techniques (e.g., hanging drops, floating spheroids, agar embedment) now constitutes the most commonly used 3D cell culture assay11-13. However, this assay is limited by the restricted set of cancer cell lines that can form spheroids and by the short period available to study cells in these conditions.
In this visualized technique, we herein introduce a sophisticated 3D cell culture assay where cancer cells of interest are embedded in a collagen gel to allow the in vitro formation of a pseudo-primary tumor that can be alternatively coated with a basement membrane-derived matrix. Once formed, the pseudo-primary tumor is then sandwiched in an acellular matrix (fibrin gel in the present case), which allows the cancer cells to cross the interface between the two matrix compartments (see Figure 1). Interestingly, secondary tumor-like structures originating from the pseudo-primary tumor along with aggressive cancer cells appear in the fibrin gel. Such a 3D culture system offers the flexibility required to investigate, for example, anticancer drugs, gene expression and cell-cell and/or cell-ECM interactions14-16.
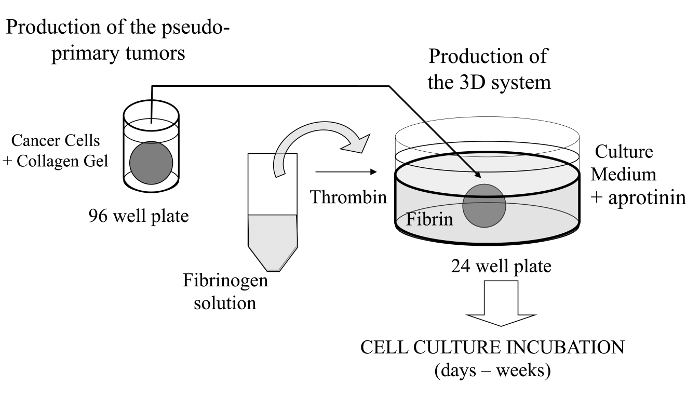
Figure 1: Overview of the Method. Schematic summary of the method to generate the 3D cell culture system as a model for cancer studies. Please click here to view a larger version of this figure.
Protocol
NOTE: No ethics consideration since animal and human cancer cells were purchased or kindly provided to us.
1. Making Collagen Plugs (Pseudo-primary Tumor)
- Prepare a collagen dispersion. Type I collagen from rat tail tendons (RTT) can be either extracted and sterilized as previously reported17, or purchased. Disperse freeze-dried RTT collagen (3.25-3.50 mg/ml in 0.02 N acetic acid) using a blender (high-speed setting; five 2 min runs) for a uniform mixing.
- Harvest (trypsin-EDTA, usually) and use trypan blue exclusion for counting viable cells using a hemocytometer. Adjust to the desired cell density (5 x 104 cells per plug).
- Prepare all solutions (NaOH, fetal bovine serum, DMEM 5x, NaHCO3) separately (Table 1) under sterile conditions and keep chilled on ice. Note: The order of addition of the various solutions is important to prevent osmotic or acidic shocks in cells.
- Perform cell dispersion (1.25 x 106 cells) into the final collagen solution (5 ml) as quickly as possible. Mix well (by pipetting up and down) while avoiding air bubbles, and then quickly distribute 200 µl of the ready-to-use solution in each well of a 96-well plate. Gently strike the multi-well plate on the work area surface of the cell culture hood to remove air bubbles and to spread the solution evenly inside the wells.
- After filling up all the wells (this step takes about 15-20 min per 96-well plate), store it into the incubator.
- Incubate the plate at 37 °C from 2 hr to overnight. Collagen gelation (i.e., fibrillogenesis) occurs within 30 min. Add culture medium (100 µl/well) to the culture to perform an overnight incubation.
2. First Layer of Fibrin Gel
- Fibrinogen Solution Preparation.
NOTE: The same batch of fibrin gel should ideally be used for more reproducible results, as fibrin gel formation may vary between different batches of commercial freeze-dried fibrinogen.- Always use a freshly prepared fibrinogen solution. Bring the freeze-dried fibrinogen to room temperature before opening the vial to avoid hydrate crystal formation.
- Progressively dissolve the fibrinogen in pre-warmed (37 °C) Hank's Balanced Salt Solution (HBSS) with Ca2+/Mg2+ at a working concentration of 3 mg/ml (consider preparing a 15% excess of the minimum final volume required: e.g., 17.25 mg in 5.75 ml for a 5 ml solution).
- Add pre-warmed HBSS dropwise at first to solubilize fibrinogen fragments. Break down larger fragments with a spatula in the beaker. Agitate the beaker from time to time to facilitate mixing. Do not use a stirrer plate during the procedure. Dissolve the remaining powder by pipetting the suspension up and down.
- Keep the fibrinogen solution lukewarm while sterilizing the solution by passing it through a 0.22 µm filter. Note: If the HBSS is not warm enough or the fibrinogen not fully dissolved, the solution may clog the filter. If applicable, replace filter once or twice, which may decrease the fibrinogen concentration, and thus fibrin clot stiffness.
- Suspend the cells of interest (e.g., endothelial cells) into ready-to-use fibrinogen solution while adjusting its final volume, as an alternative procedure.
- Preparing the Thrombin Solutions.
- Prepare a stock solution in ddH2O (50 NIH units/ml), then sterilize it using a 0.22 µm filter.
- Use a fibrinogen/thrombin ratio ≥1:0.0075 (v/v) in order to generate the fibrin gel.
- Generating the Fibrin Gel.
- Keep the sterile fibrinogen and thrombin stock solutions on ice during all the next steps. The fibrin gels are allowed to form in 24-well plates.
- Promptly overlay the surface of each well with the fibrinogen solution (200 µl/well) while avoiding air bubble formation. Process 6 wells at a time.
- Once the fibrinogen solution completely covers the surface of the wells, tilt the plate at a 45° angle and add 1.5 µl of thrombin solution to the first well by dropping the thrombin into the center of the well, and then gently swirl the plate horizontally for 1-2 sec.
- Leave the plate in a stable position under the laminar flow hood (5-10 min) until the gelation/clotting process has completed (N.B.: the polymerization process must not be disturbed, e.g., by transporting the plate to the incubator).
- Once the first six wells have polymerized, repeat the same sequence (i.e. the 3 previous steps) for the next six wells until all wells have been processed.
3. Second Layer of Fibrin Gel and Sandwiched Collagen Plug
- Option A: (Using the Collagen Plug Immediately).
- Make sure that the first layer of fibrin gel has polymerized in all wells by delicately tilting the plate. Place the 96-well plate containing the collagen gel plugs side by side with the 24-well plate (containing the fibrin gels) to ease transfer of the collagen plugs.
- Add one drop of HBSS into each well of the plate containing the collagen plugs.
- Remove each collagen plug from the well with a thin needle mounted on a syringe (used as a handle) or using a micro-spoon (see video). Transfer each collagen plug onto the first fibrin gel layer using one or two micro-spoons, while making sure that the collagen plug is well centered into the well and that sterility is well maintained.
- Overlay the previously formed fibrin gel with the second layer of fibrinogen solution (300 µl/well) and introduce the thrombin as described in 2.3, keeping a minimal 1:0.0075 ratio and a sequence of six wells at a time.
- Option B (Coating the Collagen Plug with a Thin Layer of Growth Factor-reduced Basement Membrane (GFRBM)).
- Cool all the prepared solutions and instruments beforehand and keep them at 4 °C or on ice (e.g., pipettes, tips, test tubes) during handling since frozen aliquots of GFRBM are very sensitive to excessive heating rate during thawing (follow the manufacturer's instructions).
- Following removal from the plate wells, soak each collagen plug for 2 min in a 1.5-ml centrifuge tube on ice containing 100 µl of a pure GRFBM solution.
- Transfer each coated plug onto the first fibrin layer while ensuring it is well-centered, as described earlier. Incubate the plug-containing plates at 37 °C for 5 min to allow the GRFBM to form a gel. Add the second fibrin layer as in step 3.1.4.
4. Cell Culture Medium Conditions
- Fill each well with culture medium (400 µl). The culture media and supplements will be selected based on the cell line and experimental conditions.
- Add aprotinin, an antifibrinolytic agent, to culture medium at a final concentration of 100 kallikrein inhibitor units (KIU)/ml.
NOTE: Store the plates in a cell culture incubator under the conditions used for the cell line tested. - Replenish cultures with fresh medium every other day or according to the experimental schedule, and add aprotinin. Before adding fresh medium, slightly tilt the plate (at a 30-35° angle) and incline the pipet against the side of the well while carefully suctioning the conditioned medium under constant observation.
Results
As previously mentioned, an interesting feature of this 3D cell culture assay is that cancer cells can not only migrate from the collagen plug to the adjacent fibrin gel, but also establish secondary tumors (e.g., satellite tumor-like structures). This can be directly observed with an inverted phase contrast microscope at low and high magnifications through the gel thickness, especially with a long working distance condenser (Figure 2). Using this 3D cell culture...
Discussion
As an important technical footnote, it is essential that no gap is present at the interface between the central and the peripheral gels. Otherwise, it might reduce the capacity of the cells to migrate/invade the fibrin gel. A space between the collagen and the fibrin gels may form during the first 24 hr of culture if thrombin has not been appropriately diluted. It is also possible that the cell line tested might lead the collagen gel to contract during culture, thereby causing a relatively large space to form between bot...
Disclosures
The authors have no disclosure.
Acknowledgements
Work partially funded by Prostate Cancer Canada (grant # D2014-4 to SG and CJD) and the Canadian Institutes of Health Research (grant # MOP-111069 to SG). We would like to thank Dr. Richard Poulin for editorial assistance and Mrs. Chanel Dupont for technical assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Freeze-dried collagen | Sigma-Aldrich | C7661 | from rat tail tendon (soluble dispersion) or home-made (see Rajan et al., ref.#14) |
| Fibrinogen (freeze-dried) | Sigma-Aldrich | F8630 | Type I-S, 65 - 85% protein with ≥ 75% of protein is clottable |
| Thrombin | EMD Chemicals Inc. | 605157 | Gibbstown, NJ; NIH units/mg dry weight |
| Growth factor-reduced Matrigel | Corning | 356234 | Previously from BD Biosciences |
| Aprotinin | Sigma-Aldrich | A6279 | solution at 5 - 10T IU/ml (Trypsin Inhibitor Unit) |
| Micro-spoons | Fisher Scientific | 2140115 | Fisherbrand Handi-Hold Microspatula |
| 96 well plate, round base | Sarstedt | 3925500 | |
| 24 well plate | Sarstedt | 3922 | |
| Dulbecco's modified Eagle's Medium | Sigma Chemical, Co. | D5546 | DMEM |
| Fetal Bovine Serum | VWR | CAA15-701 | FBS, Canadian origin. |
| Trypsin-EDTA | Sigma Chemical, Co. | T4049 | |
| Hank’s Balanced Salt Solution | Sigma Chemical, Co. | H8264 | HBSS |
References
- Alizadeh, A. M., Shiri, S., Farsinejad, S. Metastasis review: from bench to bedside. Tumour Biol. 35 (9), 8483-8523 (2014).
- Roudsari, L. C., West, J. L. Studying the influence of angiogenesis in in vitro cancer model systems. Adv Drug Deliv Rev. , (2016).
- Bill, R., Christofori, G. The relevance of EMT in breast cancer metastasis: Correlation or causality. FEBS Lett. 589 (14), 1577-1587 (2015).
- Kimlin, L. C., Casagrande, G., Virador, V. M. In Vitro Three-Dimensional (3D) Models in Cancer Research: an Update. Mol Carcinog. 52 (3), 167-182 (2013).
- Obenauf, A. C., Massagué, J. Surviving at a Distance Organ-Specific Metastasis. Trends Cancer. 1 (1), 76-91 (2015).
- Sykes, J. A., Fogh, J. Separation of Tumor Cells from Fibroblasts. Human Tumor Cells In Vitro. 1, 1-22 (1975).
- Lang, S. H., Stark, M., Collins, A., Paul, A. B., Stower, M. J., Maitland, N. J. Experimental Prostate Epithelial Morphogenesis in Response to Stroma and Three-Dimensional Matrigel Culture. Cell Growth Differ. 12 (12), 631-640 (2001).
- Debnath, J., Muthuswamy, S. K., Brugge, J. S. Morphogenesis and Oncogenesis of MCF-10A Mammary Epithelial Acini Grown In Three-Dimensional Basement Membrane Cultures. Methods. 30 (3), 256-268 (2003).
- Shaw, L. M. Tumor cell invasion assays. Methods Mol. Biol. 294, 97-105 (2005).
- Nelson, C. M., Bissell, M. J. Modeling Dynamic Reciprocity: Engineering Three-Dimensional Culture Models of Breast Architecture, Function, and Neoplastic Transformation. Semin Cancer Biol. 15 (5), 342-352 (2005).
- Hedlund, T. E., Duke, R. C., Miller, G. J. Three-Dimensional Spheroid Cultures of Human Prostate Cancer Cell Lines. Prostate. 41 (3), 154-165 (1999).
- Le, V. M., Lang, M. D., Shi, W. B., Liu, J. W. A Collagen-Based Multicellular Tumor Spheroid Model for Evaluation of the Efficiency of Nanoparticle Drug Delivery. Artif. Cells Nanomed Biotechnol. 15, 1-5 (2014).
- Neto, A. I., et al. A Novel Hanging Spherical Drop System for the Generation of Cellular Spheroids and High Throughput Combinatorial Drug Screening. Biomater Sci. 3 (4), 581-585 (2015).
- Janvier, R., Sourla, A., Koutsilieris, M., Doillon, C. J. Stromal Fibroblasts are Required for PC-3 Human Prostate Cancer Cells to Produce Capillary-like Formation of Endothelial Cells in a Three-dimensional Co-culture System. Anticancer Res. 17 (3A), 1551-1557 (1997).
- Doillon, C. J., Gagnon, E., Paradis, R., Koutsilieris, M. Three-dimensional Culture System as a Model for Studying Cancer Cell Invasion Capacity and Anticancer Drug Sensitivity. Anticancer Res. 24 (4), 2169-2177 (2004).
- Gobeil, S., Zhu, X., Doillon, C. J., Green, M. R. A Genome-Wide shRNA Screen Identifies GAS1 as a Novel Melanoma Metastasis Suppressor Gene. Genes Dev. 22 (21), 2932-2940 (2008).
- Rajan, N., Habermehl, J., Coté, M. F., Doillon, C. J., Mantovani, D. Preparation Of Ready-To-Use, Storable And Reconstituted Type I Collagen From Rat Tail Tendon For Tissue Engineering Applications. Nat Protoc. 1 (6), 2753-2758 (2007).
- Horie, M., et al. Characterization of Human Lung Cancer-associated Fibroblasts in Three-dimensional In Vitro Co-culture Model. Biochem Biophys Res Commun. 423 (1), 158-163 (2012).
- Banyard, J., et al. Identification of Genes Regulating Migration and Invasion Using a New Model of Metastatic Prostate Cancer. BMC Cancer. 30 (14), 387 (2014).
- Palumbo, J. S., Degen, J. L. Fibrinogen and Tumor Cell Metastasis. Haemostasis. 31, 11-15 (2001).
- Dvorak, H. F. Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J. 21 (4), 237-243 (2015).
- Luoto, K. R., Kumareswaran, R., Bristow, R. G. Tumor Hypoxia as a Driving Force in Genetic Instability. Genome Integr. 4 (1), 5 (2013).
- Das, V., Bruzzese, F., Konečný, P., Iannelli, F., Budillon, A., Hajdúch, M. Pathophysiologically Relevant In Vitro Tumor Models for Drug Screening. Drug Discov Today. 20 (7), 848-855 (2015).
- Longati, P., et al. 3D Pancreatic Carcinoma Spheroids Induce a Matrix-rich, Chemoresistant Phenotype Offering a Better Model for Drug Testing. BMC Cancer. 13 (95), (2013).
- Tan, P. H., Chia, S. S., Toh, S. L., Goh, J. C., Nathanm, S. S. Three-dimensional Spatial Configuration of Tumour Cells Confers Resistance to Chemotherapy Independent of Drug Delivery. J Tissue Eng Regen Med. , (2013).
- Koutsilieris, M., Reyes-Moreno, C., Choki, I., Sourla, A., Doillon, C., Pavlidis, N. Chemotherapy Cytotoxicity of Human MCF-7 and MDA-MB 231 Breast Cancer Cells is Altered by Osteoblast-Derived Growth Factors. Mol Med. 5 (2), 86-97 (1999).
- Lang, N. R., et al. Biphasic Response of Cell Invasion to Matrix Stiffness in Three-Dimensional Biopolymer Networks. Acta Biomater. 13, 61-67 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved