A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Flow-through Exposure System for Evaluating Suspended Sediments Effects on Aquatic Life
In This Article
Summary
A robust and flexible flow-through exposure system designed to maintain sediment in suspension is presented. The system is used to investigate the effects of suspended sediment on various aquatic species and life stages in the laboratory.
Abstract
This paper describes the Fish Larvae and Egg Exposure System (FLEES). The flow-through exposure system is used to investigate the effects of suspended sediment on various aquatic species and life stages in the laboratory by using pumps and automating delivery of sediment and water to simulate suspension of sediment. FLEES data are used to develop exposure-response curves between the effects on aquatic organisms and suspended sediment concentrations at the desired exposure duration. The effects data are used to evaluate management practices used to reduce the interactions between aquatic organisms and anthropogenic causes of suspended sediments. The FLEES is capable of generating total suspended solids (TSS) concentrations as low as 30 to as high as 800 mg/L, making this system an ideal choice for evaluating the effects of TSS resulting from many activities including simulating low ambient levels of TSS to evaluating sources of suspended sediments from dredging operations, vessel traffic, freshets, and storms.
Introduction
Dredging operations use mechanical methods to remove bottom sediments from harbors and navigation channels. During removal, some portion of the disturbed sediment is suspended into the water column, potentially making this a source of physical stress to aquatic species. In addition to being suspended, the sediment may be transported away from the dredge by ambient conditions before settling out of the water column. The combination of these two mechanisms means that an aquatic organism occurring near an operating dredge may be exposed to suspended sediments and suffer adverse effects. To address such concerns, environmental windows (seasonal dredging restrictions) are routinely used as a management practice to reduce or eliminate risk of potentially harmful impacts of suspended sediments from dredging activities on aquatic resources1,2.
Environmental windows are most commonly established to protect endangered, threatened or commercially valuable species such as the walleye (Sander vitreus) and eastern oyster (Crassostrea virginica)3. The supporting justification for imposing environmental windows often focuses on how dredge activities may potentially physically disturb (e.g., suspended sediment) an animal's ability to complete a specific part of its life history. The life stages commonly cited are eggs and larvae for keeping migration routes open for anadromous species3. However, there is limited information concerning species-specific biological effects relevant to suspended sediment4,5 available to inform using environmental windows as a risk management tool.
For these reasons, the FLEES was designed, built, and used to simulate the suspension of sediment, and to determine its effects on early life stages of aquatic organisms. FLEES studies use fine-grained sediment particles (i.e., predominantly silts, clays, and fine sands) which are most likely to remain in suspension and migrate furthest from the source. The FLEES is capable of testing fish eggs and larvae, but it can also be retrofitted to accommodate other aquatic organisms, making it a unique capability. The resulting biological response data can then be used to assess the effects of suspended sediments. The following procedures provide an overview of how the technology can be constructed and operated to yield repeatable suspended sediment concentrations and effects data using various aquatic species.
Protocol
All FLEES experiments with vertebrates were performed under the appropriate Engineer Research and Development Center (ERDC) Environmental Laboratory Institutional Animal Care and Use Protocols.
1. FLEES Modules, Water Bath, and Aquaria
- Obtain wood posts, studs and plywood for building the module. Construct the modules (in number and size) similar to a basic workbench to meet research objectives.
- Cut the plywood (0.127 cm) for the top and shelf. Cut the posts (10.16 x 10.16 cm) for the legs. For the top, cut the studs (5.08 x 10.16), build a frame and fasten the plywood to the frame. Cut a notch at the top of each leg to create a ledge and bolt the top frame to the legs.
- For the shelf, cut the studs (5.08 x 10.16), build a frame and fasten plywood (0.127 cm) to the frame. Cut a notch out of the legs 45 cm from the bottom and bolt the shelf frame to the legs. Ensure assembly is square and level.
- Obtain water bath tanks from a fiberglass tank manufacturer specializing in aquaculture tanks. Fit into the module stand a tank no larger than 152 cm long x 91 cm wide x 61 cm high. Incorporate two 2.54 cm polyvinyl chloride (PVC) slip couplings on one end of the tank by fiber glassing the couplings flush with the inside of the tank bottom.
- Place tank onto the constructed module stand with the tank drains facing the end of the stand water will drain (Figure 1). Mark on the plywood floor of the stand where the tank holes are located.
- Push the tank back and using a 3.175 cm hole saw cut two holes in the plywood for the tank drains. Slide the tank back so the drains sit in the cut holes. Connect one of the tank drains to a floor drain and the other to a water chiller heat exchanger.
NOTE: This section assumes a sanitary sewer drain is already in place.
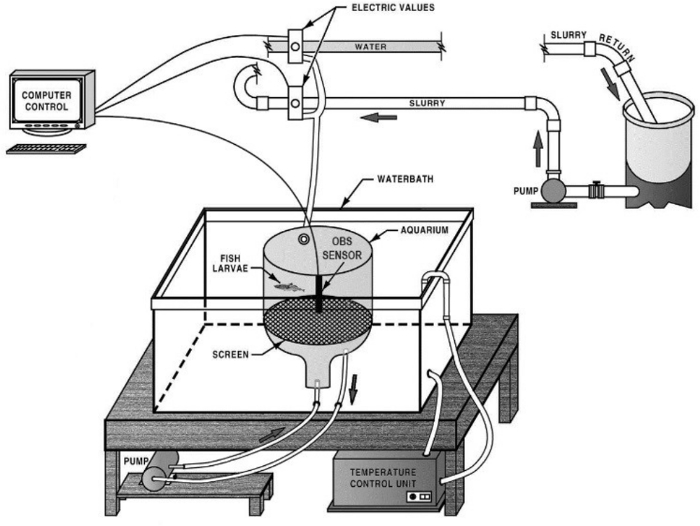
Figure 1. Schematic diagram of the Fish Larvae and Egg Exposure System (FLEES). The FLEES is modular and so is transportable. Please click here to view a larger version of this figure.

Figure 2. Polyethylene tank. A 19 L domed bottom polyethylene tank showing the overflow drain (top, with screen insert; 1.3.1; 5.6.1), slurry water inlet (right elbow; 1.3.2), pump outlet (center bottom; 1.3.3), pump inlet (off-center bottom; 1.3.4), OBS probe and clamp (4.1), and bottom screen (black ring on bottom; 5.6.1). Please click here to view a larger version of this figure.
- Obtain a 19 L domed bottom polyethylene tank (27.9 cm diameter x 36.2 cm height).
- To construct an overflow drain, use a hole saw and cut a 2.54 cm diameter hole 5 cm from the top of the tank. Install a bulkhead fitting and an insert on the exterior of the bulkhead to serve as the overflow drain.
- To construct the slurry/water inlet, use a hole saw and cut another 2.54 cm diameter hole 5 cm from the top of the aquarium. Install another bulkhead fitting and a threaded elbow hose barb (Figure 2).
- To construct the pump outlet, use a hole saw and cut a 2.54 cm diameter hole through the middle of the tank bottom and install a bulkhead fitting. Thread the exterior side of the bulkhead with an elbow hose fitting.
- To construct the pump inlet, use a hole saw and cut another 2.54 cm diameter hole located off center of the tank bottom and install a bulkhead fitting. Thread the exterior side of the bulkhead with an elbow hose fitting.
- On the exterior side of the water bath tank, measure 9 cm from the bottom and draw a line along the length of the tank. Following the line and with a hole saw, cut a pair of 2.54 cm diameter holes along the length of the water bath for each aquarium (10 total holes; distribute evenly). Install bulkhead fittings.
- Obtain magnetic drive pumps (maximum flow rate 28 L/min) for recirculating water in aquaria and suspending sediments. Mount the pumps to a stand that will fit under the water bath along the side containing the holes for connecting to the aquaria. Install an inline cord switch for each pump or wire the pumps to a switch box for power.
- Thread the exterior side of the water bath tank bulkheads with hose barbs. Attach vinyl tubing to the pump inlet and outlet and connect it to the bulkheads going to the appropriate aquarium. Inside the water bath install a quick disconnect insert into the bulkhead.
- Place the aquaria into the water bath in two rows; with three aquaria in one row arranged along the length of the water bath and the remaining aquaria in the second row (Figure 3).
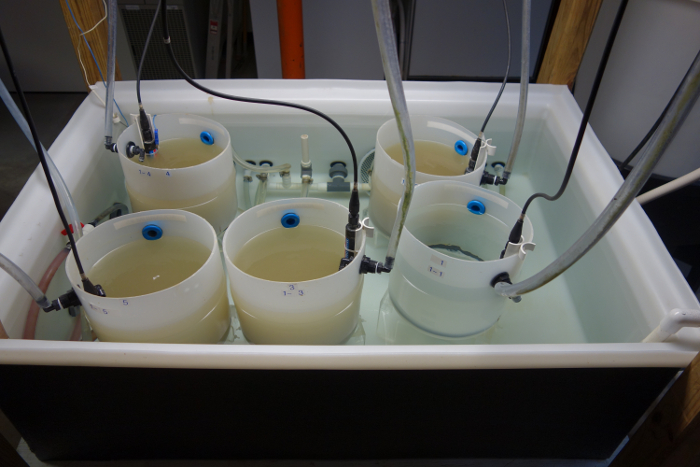
Figure 3. Water bath. Overview of a water bath with five aquaria arranged in two rows. Please click here to view a larger version of this figure.
- Connect each aquarium to a pump. Attach vinyl tubing to the hose barbs installed on the bottom of the aquaria and attach to quick disconnect valve hose barb. Connect the quick disconnects between the pump and aquarium. Install a ball valve in this connection to isolate the pump for maintenance purposes.
- Connect each aquarium's overflow drain to a common drain via vinyl tubing. Connect the common drain to the water bath drain.
- Connect each aquarium's slurry/water inlet to the slurry and water system installed on top of the module.
- Mount two light-emitting diode fixtures, designed for aquarium use, approximately 60 cm above the aquaria in each module. Use a light controller (wirelessly connected to the lights) to vary the light intensity, light color, and light cycle (e.g., 16 h light:8 h dark) to meet experimental requirements.
- Install a timer in the laboratory to control ambient lighting.
2. Slurry System
- Place one 450 L cone-bottom polyethylene tank with cover and stand at the end of the last module in line (either end can be used) to serve as the slurry reservoir. Mount a small submersible pump inside the tank to create the sediment/water slurry. Install a water chiller heat exchanger adjacent to the tank to control slurry temperature. Using a hole saw, cut a 2.54 cm hole in the tank cover to provide access to a turbidity sensor for monitoring the slurry (Figure 4).

Figure 4. Slurry tank. Cone-bottom slurry tank with cover and poly stand. Slurry water temperature is controlled by water chiller located on the floor left of the stand. The tank is connected to an air operated double-diaphragm pump (left foreground) to provide slurry to each aquarium (2.2). Please click here to view a larger version of this figure.
- Mount an air-operated double-diaphragm pump on a stand next to the slurry tank. Connect the slurry tank drain to the pump inlet. Incorporate a PVC tee (to direct slurry to the pump or to the laboratory drain) and valves to connect the tank to the pump to isolate the tank and the pump for maintenance. To power the pump, connect it to the laboratory building's air compressor.
- To provide slurry to each aquarium upon demand, mount PVC pipe on top of the modules and create a recirculation line. At the point of use located farthest from the slurry reservoir, install a return line to transport unused slurry back to the reservoir. Use flexible PVC and union fittings to connect between modules.
- Connect the slurry solenoid valves to the recirculating slurry pipe using tees, ball valves and union fittings, to isolate solenoids from the main PVC pipe for maintenance. Ensure the solenoid valves are located above the aquarium it will be supplying.
- Using a hole saw, cut 2.54 cm diameter holes in the top of the module to connect each solenoid valve to the appropriate aquarium.
- Connect the PVC pipe mounted on top of module near the slurry tank to the air-operated pump using flexible PVC and union fittings. Connect the return line to the top of the slurry tank.
- To adjust the amount of slurry introduced by the solenoid valve, install a water pressure regulator in the return line. Adjust to create the desired pressure.
3. Water System
- Install a second polyethylene tank of the appropriate volume (e.g., 500 L) with cover and stand to serve as a water reservoir. Install a water chiller heat exchanger adjacent to the tank to control water temperature. Mount a magnetic drive pump next to the water tank. Connect the water tank and pump as described in Section 2.2.
- To provide water to each aquarium upon demand, mount PVC pipe on top of the modules and create a recirculation line. Mount the PVC pipe higher than the slurry recirculating pipe. At the point of use located farthest from the water reservoir, install a return line to transport unused water back to the reservoir. Use flexible PVC and union fittings to connect between modules.
- Connect the water solenoid valves to the recirculating water pipe using tees, ball valves, and union fittings to isolate solenoids from the main PVC pipe for maintenance. Mount the solenoids behind and higher than the slurry solenoid valve. Connect the water solenoid valve to the slurry solenoid valve via vinyl tubing and hose barb fittings so that when the water solenoid turns on it will wash out remaining slurry from the line.
- Connect the PVC pipe mounted on top of the module near the water reservoir to the water pump using flexible PVC and union fittings. Connect the return line to the top of the water reservoir.
- To adjust the amount of water introduced by the solenoid valve, install a water pressure regulator in the return line. Adjust to create the desired pressure.
4. Sensors, Data Acquisition, Instrument Control and Automation
- Install an optical backscatter sensor (OBS) in each aquarium next to the slurry/water inlet to measure turbidity (Nephelometric Turbidity Units, NTU). Position the sensor so that it is submerged about 5 cm below the water surface with the sensor facing towards the middle of the tank. . Use a clamp or other device to mount the sensor.
- Using a hole saw, drill at least two 2.54 cm diameter access holes in the top of each module stand to allow access of OBS cords to electrical junction boxes mounted on top of each module.
- Install an OBS in the slurry reservoir and position the sensor so that it is completely submerged about 20 cm below the water surface.
- Wire the water and slurry solenoid valves, the OBSs located in each aquarium and slurry tank, and a thermocouple located in each water bath, into electrical junction boxes mounted on top of the module and to a data acquisition device. Install quick disconnects at the terminal ends of all wiring wherever possible.
- Use a system-design platform and development environment to design a computer application for data acquisition, instrument control and automation6. With this program, design an application to integrate the OBS and solenoid valves for measuring turbidity and introducing slurry and water into each aquarium.
- To create a variety of NTU exposure regimes, design the program to create individual profiles for each aquarium6. Create a tab and graphical user interface (GUI) for programing aquarium profiles. Label the tab 'Profiles'.
- Program the software to control exposure duration in minutes for each aquarium. Incorporate a loop sequence to continuously repeat exposure duration instructions until a certain condition is reached such as length of time. Incorporate an iteration to control how many times the loop will repeat before ending or moving onto the next set of instructions.
- Program the software to set an NTU level in each aquarium. Integrate the NTU level into the same loop/iteration sequence controlling exposure duration. Use this feature to create a variety of exposure regimes (e.g., continuous, pulsed, or no exposure) for specified durations.
- Program the software to control the opening time of the water system solenoid valves for introducing water into each aquarium in seconds (e.g., 10 s for aquarium 1, 25 s for aquarium 2, etc.). Integrate the water solenoid opening times into the loop/iteration sequence controlling exposure duration and NTU levels.
- Design the program to save all steps under 4.4 as a 'profile' for each aquarium. Include the capability to allow a user to recall saved profiles.
- Create a new tab and GUI. Label the tab 'Profile Status'. Design GUI to display a live summary of the currently loaded profile including currently active loop sequence, test time elapsed, and test time remaining.
- Create a new tab and GUI for setting water and slurry system solenoid valve values. Label the tab 'Valve Setup'.
- Program a water valve cycle interval in seconds. Design this interval as a loop sequence used to set the time between events when all water system solenoids open consecutively (in step 4.4.3 the user programmed how long each valve will remain open). Program a water valve delay in seconds. Use the delay feature to set the time between valves opening (e.g., 2 s after previous valve closed next valve will open).
- Program a slurry valve cycle interval in seconds. Design this interval as a loop sequence to set the time between events when all NTU levels measured by the OBS in each aquarium are checked against the NTU set in the aquarium profile. Check the valves and sensors consecutively. If an NTU in the aquarium is lower than the profile NTU setting then program the computer to open the slurry valve.
- Program a slurry valve opening time in seconds. Use this feature to control how long a valve remains open if slurry is needed. Program a slurry valve delay in seconds. Use the delay to set the time between valve openings.
NOTE: Ensure the cycle interval (step 4.6.1 and 4.6.2) is long enough to allow for water and slurry introductions before next loop begins. - Create buttons to manually turn on/off each water and slurry system water valve.
- Create a new tab and GUI for setting up the OBS sensors located in each aquarium and slurry tank (N=16). Label the tab 'OBS Setup'. Give each OBS a name.
- Create a feature to enter data from the OBS manufacturer's test certificate to calculate corrections for each OBS. Enter the standard NTU low (lowest NTU recorded) and standard NTU high (highest NTU recorded) as well as the voltage range for the low and high NTU.
- Create a new tab and GUI to display real time NTU measurements and NTU setting for each aquarium, as well as water temperature in each water bath. Create a button to start/stop all profiles. Create the capability to individually pause or stop an individual profile.
- Create a feature to log water temperature readings for each water bath, NTU settings and measurements for each aquarium and time stamp data into a spreadsheet. Label the tab 'Water Bath'.
5. Experimental Preparation
- Collect sediment from an area routinely dredged to maintain navigation channel depth, in close proximity to species of concern, and is known to lack historical contamination. Collect the sediment using a grab sampler or similar (e.g., Van Veen). Place sediment in 19 L plastic buckets and ship overnight on ice. Store sediments at 4 °C until use.
- Wet sieve sediment through a 1 cm screen to remove large debris; then sieve through a 450-micron stainless steel screen. Retain fine-grained (fine sands, silts, and clays) particles passing through the screen for experimental use.
- Analyze sieved sediments for chemical contamination (e.g., metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, etc. For analytical methods, see USEPA7). Characterize physicochemical parameters such as grain size distribution (percent sand silt, and clay), pH, salinity, organic carbon, and organic matter8 to meet study requirements.
- Identify the exposure duration (e.g., 72 h) and TSS concentrations (e.g., 0, 100, 250, and 500 mg/L) based on existing data or other information characterizing the suspended sediment of interest.
NOTE: Use TSS as the exposure concentration rather than NTU. TSS quantifies the mass of particles present in the water column and directly relates to physical and behavioral effects such as abrasion, loss of orientation and reduced feeding exhibited by some organisms. - Establish the NTU-TSS relationship for each FLEES aquarium.
- Turn on all FLEES hardware used for data acquisition, instrument control and automation. Randomly assign TSS treatments to FLEES aquaria using a random number table or other appropriate method. In the profile GUI, create a profile for each aquarium to execute a 72 h (4,320 min) continuous exposure using the assigned TSS concentrations generated from the random number table.
- Use professional judgment to initially program NTUs to meet TSS concentrations in each aquarium. For the control (0 mg/L TSS) set the NTU to 0; 100 mg/L TSS set the NTU to 100; 250 mg/L TSS set the NTU to 280; and 500 mg/L TSS set the NTU to 600.
NOTE: Each OBS probe will have slightly different NTU reading which is inherent to the manufacturing of the probe. - Set the opening time for water system solenoid valves to 10 s for each aquarium.
- Save profile for each aquarium.
- On the Valve Setup tab, program the water and slurry valve cycle interval. Set the water cycle interval for 600 s and water valve delay for 5 s. Set the slurry cycle interval for 180 s, opening for 3 s and delay for 1 s.
NOTE: With this program, in a 72 h test the NTUs in each aquarium will be checked 1,440 times by the computer to determine if additional slurry will be introduced and the water valves will open 432 times. Slurry valve openings are positively correlated with increasing NTUs. Typically, at 100 mg/L slurry valves open for an approximate total of 5% of the exposure duration or 72 openings; 250 mg/L ≈ 11% (158 openings); and 500 mg/L ≈ 35% (504 openings). To equal volume exchanges between aquaria adjust the opening time of the water solenoid valves for aquaria assigned lower NTUs. This will result in increased slurry valve openings at lower NTUs. - Fill the slurry tank with carbon filtered lab water. Start pump to recirculate water. In a separate container, use a mechanical mixer and homogenize test sediment.
- After the sediment is homogenized, remove a small portion (≈500 mL) and introduce into the slurry tank using a graduated polypropylene beaker. Continue to introduce sediment until 1,000 NTU is achieved.
- In the program, go to the Water Bath tab and start all aquarium profiles. Operate FLEES for at least 1 h so NTUs can stabilize in each aquarium before collecting a suspended sediment sample. Turn data logging on to record NTU readings by each aquarium OBS.
- Measure TSS using three 100 mL water samples collected from each aquarium assigned a TSS treatment < 500 mg/L. Separately measure TSS using three 50 mL water samples collected from each aquarium of a TSS treatment greater than or equal to 500 mg/L.
- Measure TSS by vacuum filtering samples through pre-weighed 0.45 µm filter paper. Immediately post-filtering, dry the filter and contents at 105 °C for a minimum of 4 h and then reweigh to the nearest 0.1 mg. Use the average of the three samples as a measure of TSS in each aquarium.
- Compare averages obtained in Section 5.5.4 to the observed NTU measurements recorded for each aquarium. Reprogram the NTU limits until the desired TSS concentration is achieved (e.g., 600 NTU ≈ 500 mg/L TSS).
- Determine mesh size of screen needed to contain animals within each aquarium.
- For larger animals such as fish (e.g., > 3 cm) or shellfish place a screen on the bottom to separate animals from the pump opening. Install a screen insert in the aquarium's overflow drain bulkhead to prevent escape.
- Contain small life stages such as fish eggs, larvae and fry in a chamber (10.16 cm diameter (i.d.) by 12.7 cm long (1,029 mL) made of PVC pipe) for submerging in a FLEES aquarium (Figure 5).
- Cut three 8.25 cm wide by 9.52 cm long holes out of the side of the chamber. Install nylon screen cloth on the bottom of the chamber and on the holes cut of the side. Use a PVC cap as a removable lid to introduce and remove test animals.
- Cut a circular hole in the cap sufficient for viewing test animals from above while leaving an edge for attaching a nylon screen cloth. Install all screens on the inside of the chamber to prevent organisms from coming into contact with the sharp PVC edges.
NOTE: Select a screen mesh size that contains test animals while enabling suspended test sediment to enter. - Completely submerge the chamber in the middle or to the side of an aquarium by suspending it vertically using three short lengths of rope (#18 white twisted mason line) and hooks constructed from electrical wire. Tie a Blake's hitch knot near each hook and adjust the length of the rope to level the chamber.
- Determine how many aquarium tank volume exchanges are required per day to meet project and water quality objectives. Adjust the water pressure regulator (see Section 2.2) and solenoid opening time (e.g., open every 10 min for 10 s) to create the desired water flow rate. Fill water baths with water and operate water chiller heat exchangers to confirm that test temperatures can be achieved and maintained.

Figure 5. FLEES sub-chamber. Overview of an exposure sub-chamber suspended in an aquarium with no added sediment (left). Fish larvae of the appropriate size can be contained within the sub-chamber to reduce the possibility of escape and injury (right). Please click here to view a larger version of this figure.
6. Experimental Procedures
- Turn on all FLEES hardware used for data acquisition, instrument control and automation. Fill the aquaria, water baths, and water reservoir with the desired test water. Start all water chiller heat exchangers. Confirm and adjust light cycle.
- Fill the slurry tank with carbon filtered tap water. Start pump to recirculate water. Use a mechanical mixer and homogenize the container of test sediment. After the sediment is homogenized, remove a small portion (≈500 mL) and introduce into the slurry tank. Continue to introduce sediment until 1,000 NTU is reached.
- In the profile GUI, create a profile for each aquarium to execute a 72 h (4,320 min) continuous exposure using the same TSS assignments used in preparation. Use data obtained during experimental preparations to program NTUs to meet TSS concentrations in each aquarium. For the control (0 mg/L TSS) set the NTU to 0; 100 mg/L TSS set the NTU to 100; 250 mg/L TSS set the NTU to 280; and 500 mg/L TSS set the NTU to 600.
- On the Valve Setup tab, use data obtained from preparation (Section 5.5.8) to program the water and slurry valve cycle interval. Set the water cycle interval for 600 s and water valve delay for 5 s. Set the slurry cycle interval for 180 s, opening for 3 s and delay for 1 s.
- Introduce animals into aquaria using guidelines set forth in the approved animal care and use protocol. For eggs, transfer from the holding tank to an exposure chamber via a plastic transfer pipette. For larger fish, such as fingerling (2-8 cm total length), use a nylon aquarium net.
- After animals are stocked into aquaria, access the GUI and in the Water Bath tab start all aquarium profiles. Operate FLEES for at least 1 h so NTUs can stabilize in each aquarium before collecting a suspended sediment sample. Turn data logging on to record NTU readings by each aquarium OBS.
- Maintain FLEES daily by topping off water and slurry reservoirs with test water and sediment.
NOTE: The frequency of slurry introductions is positively correlated with increasing NTU levels. Therefore, the amount of water and sediment used each day is dependent on programmed NTUs and desired volume exchanges. Typically, 25-50 gal may be used each day of either water or slurry.- Gently wipe OBS probes daily with a wet cloth to remove sediment build up on the sensor face. Check water chillers and pumps for normal operation. Collect concurrent TSS measurements daily to predict TSS for the remainder of the day based on NTU measurements recorded at specified intervals by the computer program.
- Measure temperature, dissolved oxygen, pH (and other parameters depending on the species and other requirements) daily for each aquarium using a hand-held multi-probe water quality instrument designed for this purpose.
- Terminate an experiment automatically by specifying exposure duration in each aquarium profile or manually by stopping all aquarium profiles.
- Determine experimental endpoints to be measured such as hatching success, time to hatch, mortality, growth (length and weight), and gross morphology.
Results
A series of operational runs is performed before beginning an experiment to ensure that the FLEES is delivering the appropriate concentrations of sediments to each aquarium (Sections 5.5 and 6.2). Figure 6 illustrates how NTU concentrations are maintained in experimental aquaria to achieve target suspended sediment concentrations. In this example, FLEES evaluated whether suspended sediment could be maintained over a three-day period with the proposed test sediment, a dura...
Discussion
The FLEES technology improves on existing methods4,9 by maintaining and controlling suspended sediments over a wide range of exposure times and suspended sediment concentrations using an automated, computer controlled system. The technology is flexible such that it can be used to evaluate the effects of suspended sediments to multiple aquatic species and life stages of varying sizes from eggs to adults depending on the species. In the future, the technology is capable of assessing suspended sediment effects to...
Disclosures
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. Citations of commercial organizations or trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations. Authors Burton C. Suedel and Justin L. Wilkens, both of the US Army Engineer Research and Development Center, declare that they have no competing financial interests.
Acknowledgements
This research was funded by the U.S. Army Corps of Engineers Dredging Operations and Environmental Research Program, Todd Bridges, Director. Permission was granted by the Chief of Engineers to publish this material.
Materials
| Name | Company | Catalog Number | Comments |
| Parts List for One FLEES Module, Water Bath, and Aquarium | |||
| post, wood - used to build module (cut to 78 in) | Local vendor | N/A | Quantity: 4 Size: 4 in x 4 in x 8 ft |
| plywood, marine grade - fastened to wooden posts about 18 in off ground - for holding water bath (60 in x 42 in) | Local vendor | N/A | Quantity: 1 Size: 3/4 in x 4 ft x 8 ft |
| plywood - fastened on top of wooden posts - for holding pipes, solenoids and electrical (60 in x 42 in) | Local vendor | N/A | Quantity: 1 Size: 1/4 in x 4 ft x 8 ft |
| stud, wood - used to brace plywood and wooden posts (cut to fit) | Local vendor | N/A | Quantity: 4 Size: 2 in x 4 in x 96 in |
| tank, fiberglass - water bath with two drains: 1) to supply chiller; and 2) to drain water | Hydro Composites, LLC, Stockdale, TX, USA | FBT-226 | Quantity: 1 Size: 150-gal |
| chiller, water with self contained pump - for water bath; chiller sits under module | Remcor Products Co., Glendale Heights, IL, USA | CFF-500 | Quantity: 1 Size: 1/2 hp |
| tank, domed bottom - FLEES aquaria - sit inside water bath | United States Plastic Corp, Lima, OH, USA | 5197 | Quantity: 5 Size: 19 L |
| tank, stand - acrylic stand, 12 in x 12 in x 6 in - to hold aquaria | custom built by ERDC shops | N/A | Quantity: 5 Size: custom |
| pump, magnetic drive - to suspend sediment in each aquarium | March Manufacturing Inc., Glenview, IL, USA | MDX-3-1/2 115 v | Quantity: 5 Size: 28 liter per min |
| light, LED - installed over water bath | C2 Development, Inc., Ames, IA, USA | Hydra 26 | Quantity: 2 Size: based on area to light |
| pipe, PVC schedule 40 - installed in drain of water bath to control water level | Local vendor | N/A | Quantity: - Size: 1 in |
| fittings, bulkhead - for aquaria/water bath connections to pumps, drains, water and slurry lines | Lifegard Aquatics, Cerritos, CA, USA | R270900 | Quantity: 30 Size: 1/2 in FPT x FPT |
| fittings, quick-disconnect, male pipe threaded inserts - insert in tank bulkhead | Cole-Parmer, Vernon Hills, IL, USA | EW-31303-36 | Quantity: 10 Size: 1/2 in MPT |
| fittings, quick-disconnect, valved hose barbs - connection between aquarium and insert in tank bulkhead | Cole-Parmer, Vernon Hills, IL, USA | EW-31303-11 | Quantity: 10 Size: 1/2 in |
| fittings, black HDPE threaded elbow - for aquaria vinyl tube connections to slurry/water line and pump | United States Plastic Corp, Lima, OH, USA | 62043 | Quantity: 20 Size: 1/2 NPT x 1/2 in Hose ID |
| fittings, black HDPE threaded adapter - for connections between pump and tank bulkhead | United States Plastic Corp, Lima, OH, USA | 62017 | Quantity: 10 Size: 1/2 NPT x 1/2 in Hose ID |
| tube, vinyl - connect slurry/water line to aquaria and to connect pumps to aquaria | Local vendor | N/A | Quantity: 25 ft Size: 1/2 in ID |
| tube, vinyl - connect to aquaria drains inserts and water bath drain | Local vendor | N/A | Quantity: 25 ft Size: 5/8 in ID |
| clamp, hose, stainless steel - to clamp vinyl tube to hose barbs | Local vendor | N/A | Quantity: 40 Size: #8 |
| Parts List for Slurry System | |||
| chiller, water with self contained pump - sits off to side of slurry tank | Remcor Products Co., Glendale Heights, IL, USA | CFF-500 | Quantity: 1 Size: 1/2 hp |
| 125 gallon open top cone bottom tank w/Stand - 42 in x 35 in - contains the water and sediment to make slurry | United States Plastic Corp, Lima, OH, USA | 8586 | Quantity: 1 Size: 125 gal |
| Cover for 125 gallon tank | United States Plastic Corp, Lima, OH, USA | 8935 | Quantity: 1 Size: 42 in x 35 in |
| valve, PVC - connect tank drain to pump - isolate for maintenance | local plumbing vendor | N/A | Quantity: 2 Size: 1-1/2 in |
| pump, double diaphragm mounted on stand - used to recirculate slurry | Wilden-pumps.co.uk & Air Pumping Ltd., Essex, UK | P2/PPPP/WF/WF/PTV/400 | Quantity: 1 contact distributor |
| sensor, optical backscatter - measure NTU in slurry tank | Campbell Scientific, Logan, UT, USA | OBS-3+ | Quantity: 1 Size: 0-1,000 NTU |
| pipe, PVC Schedule 40 - to recirculate slurry | local plumbing vendor | N/A | Quantity: 20 ft Size: 1 in |
| pipe, flexible PVC - fitted with union and used to connect to next module | local plumbing vendor | N/A | Quantity: 10 ft Size: 1 in |
| union, PVC Schedle 80 Socket - connect slurry line with next module | local plumbing vendor | N/A | Quantity: 8 Size: 1/2 in |
| solenoid, plastomatic (normally closed) - introduce slurry | Plast-O-Matic Valves, Inc., Cedar Grove, NJ, USA | EASYMT4V12R24-PV | Quantity: 5 Size: 1/2 in NPT threaded, 24 VAC contact distributor |
| fitting, PVC tee - connect slurry pipe with solenoid | local plumbing vendor | N/A | Quantity: 5 Size: 1 in x 1 in x 1 in slip x slip x FIPT |
| fittings, 1 in PVC ball valve threaded - shut off for slurry delivery to solenoid/water lines | local plumbing vendor | N/A | Quantity: 7 Size: 1/2 in |
| fittings, 1 in PVC union threaded - connect slurry solenoid to shut off valve | local plumbing vendor | N/A | Quantity: 5 Size: 1/2 in |
| tube, vinyl - connection between water solenoid and slurry solenoid | Local vendor | N/A | Quantity: 50 ft Size: 1/4" ID |
| Parts List for Water System | |||
| chiller, water with self contained pump - sits off to side of reservoir | Remcor Products Co., Glendale Heights, IL, USA | CFF-500 | Quantity: 1 Size: 1/2 hp |
| 125 gallon open top cone bottom tank w/Stand - 42 in x 35 in - contains the water and sediment to make slurry | United States Plastic Corp, Lima, OH, USA | 8586 | Quantity: 1 Size: 125 gal |
| Cover for 125 gallon tank | United States Plastic Corp, Lima, OH, USA | 8935 | Quantity: 1 Size: 42 in x 35 in |
| valve, PVC - connect tank drain to water pump | local plumbing vendor | N/A | Quantity: 2 Size: 1 in |
| pump, magnetic drive, in-line use - used to recirculate water to aquaria and chiller | Little Giant, Fort Wayne, IN, USA | 3-MD-SC | Quantity: 1 Size: 1/12 hp |
| solenoid, alco - introduce water | discontinued; ASCO, Florham Park, NJ,USA for similar | N/A | Quantity: 5 Size: 24 v, 1/4 in NIPT |
| fittings, black HDPE reducer connector - connect 1/4 in hose water line from solenoid to 1/2 in hose | local plumbing vendor | N/A | Quantity: 5 Size: 1/2 in hose ID x 1/4 in hose ID |
| fittings, black HDPE tee - connect 1/2 in hose water line and slurry to aquaria | local plumbing vendor | N/A | Quantity: 5 Size: 1/2 in NPT x 1/2 in hose ID x 1/2 in hose ID |
| fittings, street elbow | local plumbing vendor | N/A | Quantity: 5 Size: 1/2 in 90° MIPT x FIPT |
| fittings, PVC threaded pipe nipples - connect union fittings with solenoids and other connections | local plumbing vendor | N/A | Quantity: 12 Size: 1/2 in |
| fittings, union threaded - connect slurry/water lines with next module | local plumbing vendor | N/A | Quantity: 6 Size: 1 in PVC |
| fittings, reducer bushing - connect to reducer tee in water line | local plumbing vendor | N/A | Quantity: 5 Size: 1/2 in male by 1/4 in female FIPT |
| fittings, threaded pipe nipples - connection between bushing and water solenoid | local plumbing vendor | N/A | Quantity: 5 Size: 4 in long x 1/4 in |
| pipe, PVC - make connections between tank, pump and chiller | local plumbing vendor | N/A | Quantity: 5 ft Size: Schedule 40 |
| Parts List for Sensors, Data Acquisition Device, and Computer Software | |||
| software, LabView | National Instruments, Austin, Texas, USA | LabView 2015 Base | Quantity: 1 Size: N/A |
| SCXI-1001 12-Slot Chassis, U.S. 120 VAC | National Instruments, Austin, Texas, USA | 776571-01 | Quantity: 1 Size: N/A |
| SCXI 1100 - 32-Channel, ±10 V Analog Input Module | National Instruments, Austin, Texas, USA | 776572-00 | Quantity: 1 Size: N/A |
| SCXI 1303 - Terminal block designed for high-accuracy thermocouple measurements | National Instruments, Austin, Texas, USA | 777687-03 | Quantity: 2 Size: N/A |
| SCXI 1102B - 32-Channel Thermocouple/Voltage Input Module | National Instruments, Austin, Texas, USA | 776572-02B | Quantity: 1 Size: N/A |
| SCXI 1161 - General-Purpose Relay Module | National Instruments, Austin, Texas, USA | 776572-61 | Quantity: 6 Size: N/A |
| SCXI 1300 - General-Purpose Voltage Module | National Instruments, Austin, Texas, USA | 777687-00 | Quantity: 1 Size: N/A |
| PCMCIA Card DAQCARD-AI-16E-4 | National Instruments, Austin, Texas, USA | N/A - legacy | Quantity: 1 Size: N/A used cards available online |
| sensor, optical backscatter - measure NTU in each aquarium | Campbell Scientific Inc., Logan, UT, USA | OBS-3+ | Quantity: 5 Size: 0-1,000 NTU |
References
- National Research Council (NRC). A process for setting, managing, and monitoring environmental windows for dredging projects. Marine Board, Transportation Research Board, Special Report 262. , (2001).
- Suedel, B. C., Kim, J., Clarke, D. G., Linkov, I. A risk-informed decision framework for setting environmental windows for dredging projects. Sci Total Environ. 403, 1-11 (2008).
- Reine, K. J., Dickerson, D. D., Clarke, D. G. Environmental windows associated with dredging operations. DOER Technical Notes Collection. ERDC TN DOER-E2. , (1998).
- Wilber, D. H., Clarke, D. G. Biological effects of suspended sediments: A review of suspended sediment impacts on fish and shellfish with relation to dredging activities in estuaries. N Am J Fish Manag. 21, 855-875 (2001).
- Suedel, B. C., Lutz, C. H., Clarke, J. U., Clarke, D. G. The effects of suspended sediment on walleye (Sander vitreus) eggs. J. Soils Sediments. 12, 995-1003 (2012).
- Travis, J., Kring, J. . LabVIEW for Everyone: Graphical Programming Made Easy and Fun (National Instruments Virtual Instrumentation Series). , (2006).
- Hazardous Waste Test Methods/SW-846 On-line. Office of Solid Waste and Emergency Response (OSWER) Available from: https://www.epa.gov/hw-sw846/sw-846-compendium (2016)
- Plumb, R. H. . Procedure for handling and chemical analysis of sediment and water samples, EPA/CE-81-1. , (1981).
- Clarke, D. G., Wilber, D. H. . Assessment of potential impacts of dredging operations due to sediment resuspension. , (2000).
- Suedel, B. C., Clarke, J. U., Lutz, C. H., Clarke, D. G., Godard-Codding, C., Maul, J. Suspended sediment effects on walleye (Sander vitreus). J. Great Lakes Res. 40, 141-148 (2014).
- Wilkens, J. L., Katzenmeyer, A. W., Hahn, N. M., Hoover, J. J., Suedel, B. C. Laboratory test of suspended sediment effects on short-term survival and swimming performance of juvenile Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus). J. Appl. Ichthy. 31, 984-990 (2015).
- Suedel, B. C., Clarke, J. U., Wilkens, J., Lutz, C. H., Clarke, D. G. The effects of a simulated sediment plume on eastern oyster (Crassostrea virginica) survival, growth, and condition. Estuaries and Coasts. 38 (2), 578-589 (2015).
- Bilotta, G. S., Brazier, R. E. Understanding the influence of suspended solids on water chemistry and aquatic biota. Water Res. 42, 2849-2861 (2008).
- Reine, K., Clarke, D., Dickerson, C., Pickard, S. Assessment of potential impacts of bucket dredging plumes on walleye spawning habitat in Maumee Bay, Ohio. Proceedings of the 18th World Dredging Congress (WODCON XVIII). , (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved