A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Experimental Protocol of a Three-minute, All-out Arm Crank Exercise Test in Spinal-cord Injured and Able-bodied Individuals
In This Article
Summary
We present a protocol to test the aerobic and anaerobic power of the upper body muscles over a duration of 3 min in able-bodied as well as in paraplegic and tetraplegic individuals. The protocol presents specific modifications in its application for upper-body exercise in individuals with or without disability.
Abstract
Reliable exercise protocols are required to test changes in exercise performance in elite athletes. Performance improvements in these athletes may be small; therefore, sensitive tools are fundamental to exercise physiology. There are currently many exercise tests that allow for the examination of exercise capacity in able-bodied athletes, with protocols mainly for lower-body or whole-body exercise. There is a trend to test athletes in a sport-specific setting that closely resembles the actions that the participants are used to performing. Only a few protocols test short-term, high-intensity exercise capacity in participants with an impairment of the lower body. Most of these protocols are very sport-specific and are not applicable to a wide range of athletes. One well-known test protocol is the 30 s Wingate test, which is well-established in cycling and in arm crank exercise testing. This test analyzes high-intensity exercise performance over a 30 s time duration. In order to monitor exercise performance over a longer duration, a different method was modified for application to the upper body. The 3 min, all-out arm crank ergometer test allows athletes to be tested in a manner specific to 1,500 m wheelchair racing (in terms of exercise duration), as well as to upper body exercises such as rowing or hand-cycling. In order to increase the reliability with identical test conditions, it is crucial to precisely replicate settings such as the resistance (i.e., torque factor) and the position of the participants (i.e., the height of the crank, the distance between the crank and the participant, and the fixation of the participant). Another important issue concerns the beginning of the exercise test. Fixed revolutions per minute are required to standardize the test conditions for the start of the exercise test. This exercise protocol shows the importance of accurate operations to reproduce identical test conditions and settings.
Introduction
There are several exercise tests that accurately determine the increase in exercise performance in elite athletes over the course of a training period1,2,3,4,5. One of these tests is the reliable 3-min all-out exercise test on a braked cycling ergometer3,4,5,6. This test was used to determine critical power, but it was also applied to exercise testing with athletes, as well as to research7,8,9. As this test was mainly used for lower-extremity performance, such as in rowing7 and cycling3,5, a similar testing protocol for upper-body exercise was needed. Sport disciplines that mainly use the upper body might be possible beneficiaries for such a new test protocol, in addition to athletes or individuals with an impairment of lower body muscles (e.g., an amputation or an impairment of limbs due to a spinal cord injury). Hence, a test protocol on the arm crank ergometer is a good tool to easily test upper-body exercise performance in a variety of athletes from different sport disciplines.
The existence of a very similar 30 s Wingate arm crank ergometer test10,11 helped with the development of a protocol for a 3 min, all-out arm crank ergometer test. Its duration is very similar to that of a 1,500 m wheelchair race. Therefore, this new test protocol of the 3 min, all-out arm crank ergometer test was tested for its test-retest reliability12. Overall, the reliability of this test protocol was excellent, so it could be a future testing tool in the field of upper-body exercise testing. Nevertheless, the use of this exercise test requires attention, especially when testing individuals with a spinal cord injury. Therefore, the aim of this experimental article is to demonstrate a detailed protocol that describes not only the test settings and analysis of the test results, but that also indicates the differences between testing able-bodied individuals and athletes with a spinal cord injury.
Protocol
The study was approved by the local ethical committee (Ethikkommission Nordwest- und Zentralschweiz, Basel, Switzerland), and written informed consent was obtained from the participants before starting the study.
1. Test Preparation and Participant Instruction
- Arm crank ergometer
- Turn the power on the rotation speed-dependent arm crank ergometer before opening the software.
- Choose the test protocol for the 3 min, all-out ergometer test.
- Insert a new protocol with a 120 s warmup, 180 s of test duration, and a 720 s cooldown period. Choose this test protocol and open a new participant sheet.
- For every new test, determine the participant's body mass beforehand.
- Set the relative torque factor to 0.2 for able-bodied and paraplegic individuals (e.g., for a 100-kg participant with a relative torque factor of 0.2, a torque of 20 Nm results)12.
- Apply a lower torque factor for tetraplegic participants depending on the lesion level of the spinal cord injury; two or more familiarization trials are needed to determine the optimal relative torque factor for the relevant participant.
- Perform a familiarization trial in the same manner as described in section 2. If no peak appears after printing the data from the familiarization trial, or if the participant was not able to crank for the whole 3 min, perform a second familiarization trial with a lower torque factor. Give participants a rest of at least two days between each trial.
- Exercise test settings
- Adjust the height of the arm crank and record it to replicate identical test settings in the next test session. Adjust and record the distance between the arm crank ergometer and the participant.
- To determine the height, measure the distance between the floor and the fixation of the crank. To record the distance between the crank and the participant, measure and record the distance between the wall and the chair fixation. Adjust the arm crank axis to a height horizontal to the shoulder joint.
- Record either the distance between the wall fixation and the chair or the distance between the ergometer and the chair fixation. Adjust the chair settings according to whether the participant is a) able-bodied, b) paraplegic ,or c) tetraplegic.
- If the participant is able-bodied, have the participant sit in the chair provided by the distributor.
- If the participant is paraplegic and needs to sit in their own wheelchair, use a fixation set to fix the wheelchair to the arm crank ergometer. If the participant does not need their own wheelchair, have the participant sit in the chair provided by the distributor.
- If the participant is tetraplegic, fix their upper body to the chair provided by the distributor or to their own wheelchair and possibly fix their hands to the pedals. To fix the upper body, use a strap with hook-and-loop fastener. For hand fixation, use a wristband in tetraplegic patients.
- Adjust the height of the arm crank and record it to replicate identical test settings in the next test session. Adjust and record the distance between the arm crank ergometer and the participant.
- Additional measurements
- Make sure that the lactate system solution is refilled before using the lactate analyzer. Insert a new chip sensor every six months. Use the quality control solution (12 mM) every day and the 3 mM quality control solution every two weeks.
- Put the 12 mM quality control solution into the "STD 1" slot every morning.
- To further enhance the quality, add 3 mM quality control solutions into gap "1" and "2" and run a measurement by pressing "start" every two weeks. The measurement should result in a range between 2.96 and 3.10 mM.
- To determine the whole-blood lactate concentration before and after the 3 min, all-out arm crank ergometer test, obtain a baseline lactate concentration. Disinfect the earlobe with a disinfectant before drawing a blood sample from the earlobe using a 10 µL capillary. Use a lancet to get the whole blood sample.
- When the capillary is completely full of blood, put it into the hemolysis cup.
NOTE: These cups are commercially available and prefilled with a hemolyzing solution. Shake the solution until the blood is completely mixed before putting it into the tray of the lactate analyzer. - Run a calibration before analyzing the lactate concentration. Place the quality control cup into the lactate analyzer (see step 1.3.1.1.). Ensure that the calibration results in a 12-mM lactate concentration; otherwise, replace the chip sensor.
- Place the samples into the numbered slots, starting with "1" for the sample that was taken first.
NOTE: After the calibration is completed, the samples are measured automatically by the chip sensor system.
- When the capillary is completely full of blood, put it into the hemolysis cup.
- To determine the heartrate, place a heartrate belt around the chest of the participant and fix the heartrate monitor to the arm crank ergometer. Start the measurement by pressing on the red start button on the monitor. If no heartrate is displayed on the watch, wet the heartrate belt with water to ensure a good recording of the heartrate.
- To determine oxygen consumption during warmup and during the 3 min all-out test, calibrate the metabolic cart before the test. Run automatic volume and gas calibration immediately before the test and before putting the mask on.
- Open the automatic volume calibration in the software and press the start button. Store the results if the error is below 3% on the screen.
- Open the gas calibration in the software, as well as the calibration gas, and start with the automatic calibration.
NOTE: The calibration gas consists of 5% CO2, 16% O2, and 79% N2. When 8 green buttons are displayed on the screen at the end of the calibration, the calibration is successful and the results can be stored. Close the gas bottle to ensure no leakage of gas. - Ensure that the participant's actual body mass is inserted into the computer program. After the participant is chosen by the search engine on the computer, choose "ergospirometry" in the software and start with the measurement of room air concentration by pressing the start button.
- To run this calibration, take the sensor out of the spirometer and press the start button. Calibration is finished when "ok" is displayed on the computer.
- Meanwhile, during the calibration, put the oxygen mask on the participant.
- When the measurement of room air concentration is finished and the program is ready to measure, put the sensor back into the spirometer. Then, put the whole spirometer into the cavity of the mask; the device is now ready to measure oxygen consumption.
- In addition, fix the hose of the spirometer somewhere (e.g., on the shoulder with an adhesive tape) so that it does not interfere during the arm crank exercise.
- Make sure that the lactate system solution is refilled before using the lactate analyzer. Insert a new chip sensor every six months. Use the quality control solution (12 mM) every day and the 3 mM quality control solution every two weeks.
2. Execution of the Exercise Protocol
- Warmup
- 1 min before starting the warmup, start measuring the oxygen consumption at rest when the participant sits at the arm crank ergometer without moving or talking. Press the start button in the software program.
- At the same time, start the measurement of the heartrate by pressing the red button. Measure the heartrate during the warmup, as well as during and after the test.
- Perform a standardized warmup over 2 min at 20 W before the start of the test. During the last 30 s of the warmup, keep the cadence constant at 60 rpm. Count down the last 10 s of the 30-s warmup.
- 3 min all-out exercise test
- At the end of the countdown, make sure to give a clear starting signal by shouting "go." After the start signal is given, allow the participant to accelerate.
- Instruct the participant to accelerate the arm crank ergometer to the maximum possible speed right at the beginning of the test. Keep the cadence at the maximum possible speed during the whole test. For standardization reasons, do not encourage the participants during the tests.
- Give information on duration every 30 s. Finish the test after a duration of 3 min.
- Cooldown and post analysis
- After finishing the 3 min all-out test, measure the end lactate concentration, if desired, and thereafter every 2 min for the next 10 min. Re-use the same puncture site for blood sampling as was used before the test.
- Stop the measurement of oxygen consumption after finishing these 3 min by pressing the stop button. Remove the oxygen mask. Save the measurement of oxygen consumption on the computer by pressing the exit button and by clicking "yes" when the software asks for data storage.
NOTE: The data is stored in the software program and can easily be converted to a csv document later. - To export the data, press the "Export" button to convert the file to a csv document for later analysis. Stop the heartrate measurement by pressing the stop button on the left side of the heartrate monitor after all blood samples have been drawn from the earlobe.
3. Data Analysis and Interpretation of the Results
- Performance parameters
- Analyze several different parameters after finishing this performance test.
First, save the test and export it to a spreadsheet. - Calculate the mean power (Pmean =
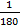
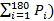 over 3 min, the peak power, and the minimal power in between these 3-min12.
over 3 min, the peak power, and the minimal power in between these 3-min12.
NOTE: the peak power (Ppeak) is the maximal power during the whole 3 min. The power is measured in 0.2-s intervals. The peak power is the highest and the minimal power (Pmin) the lowest single-power measurement. - Calculate the fatigue index as the power decline per second from the peak power to the end power ((Ppeak [W] – Pmin [W])/(tmin [s] – tpeak [s])).
- Calculate the total work over the whole 3 min by adding the work done every second (Work [J]= resistance [kg] * revolutions per min * flywheel distance [m] * time [min]).
- Calculate the time from the start to the peak power (time to peak power = tpeak [s]). Furthermore, calculate the relative peak (relative Ppeak = Ppeak/kg body mass) and the mean power (relative Pmean = Pmean/kg body mass) by dividing the absolute values by the body mass of the participant.
- Divide the 3-min all-out test into 30-s segments to check the pacing strategy and fatigue over these 3 min. Calculate the mean power for every 30-s segment (Pmean =
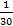
 .
.
- Analyze several different parameters after finishing this performance test.
- Other measurements
- Place all blood samples into the numbered slots of the blood lactate analyzer and run the measurements automatically by pressing "analyze." Print blood lactate concentrations for later analysis by turning on the printer.
- Transmit the heartrate measurements to the computer using an infrared device from the manufacturer. Open the software of the heartrate monitor and import the data from the heartrate monitor to the software. Store the data locally and, if desired, export it to a spreadsheet for later analysis (e.g., segment analysis)13.
- Set a marker right at the beginning of the 3 min and at the end of the 3 min, allowing the average, maximal, and minimal heartrate to be calculated automatically for this segment.
NOTE: The heartrate is averaged automatically by the software over 5 s intervals. - Export the data for oxygen consumption to a csv file (step 2.3) and open it in a spreadsheet for analysis14. Calculate the average oxygen consumption at rest: (VO2_rest =

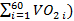 and during the 3 min (VO2_180s =
and during the 3 min (VO2_180s = 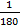
 , as well as the peak oxygen consumption and oxygen consumption during the 30-s segments: (VO2_30s =
, as well as the peak oxygen consumption and oxygen consumption during the 30-s segments: (VO2_30s = 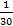
 .
.
NOTE: The data for oxygen consumption are measured breath-by-breath and then averaged automatically over a duration of 15 s per segment. The peak oxygen consumption is the highest value over a 15-s interval during the 3-min exercise test.
- Statistics
- Use the Shapiro-Wilk test, the Q-Q-plot, and the Kolmogorov Smirnov tests to check the normal distribution of the data. If the data is normally distributed, present it as the mean and the standard deviation (SD).
- Analyze the test-retest reliability using the intra-class correlation coefficient (ICC; 3,1 model)15.
- Calculate the absolute and relative reliability using the standard error of the measurement (SEM), coefficient of variation (CV), smallest real difference (SRD), and 95% confidence interval of the ICC16.
NOTE: The ICC should be interpreted according to Munro`s classification17: 0.26 to 0.49 reflects a low correlation; 0.50 to 0.69 reflects a moderate correlation; 0.70 to 0.89 reflects a high correlation; and 0.90 to 1.0 indicates a very high correlation. The absolute reliability should be presented as the SRD, CV, and SEM, and the relative reliability should be in the form of the ICC16,18. - Analyze significant changes between the two test sessions using a paired t-test. To show the agreement of the data sets of both test sessions, use Bland-Altman19 plots. Use statistical software to perform data analysis; set a statistical significance level of 0.05 throughout.
Results
The test-retest reliability was checked in 21 recreationally trained (but not specifically upper-body trained), non-smoking individuals (9 males, 12 females; age: 34 ± 11 years; body mass: 69.6 ± 11.1 kg; and height: 175.5 ± 6.9 cm). Table 1 shows the results for the relative and absolute test-retest reliabilities12. The peak power compared between the test and retest is presented in Figure 112
Discussion
Exercise testing in spinal-cord injured athletes is crucial to tracking exercise performance over several months or years of training. Only a few exercise tests exist to check short-term, high-intensity exercise performance on the arm crank ergometer. This method describes in detail how an exercise test that was already examined for its reliability in cycling5 and rowing7 might be applied to the arm crank ergometer. To collect reliable and meaningful results, two factors ar...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are grateful for the assistance from Martina Lienert and Fabienne Schaufelberger during exercise testing, as well as from P. D. Claudio Perret, PhD for his scientific advice.
Materials
| Name | Company | Catalog Number | Comments |
| Angio V2 arm crank ergometer | Lode BV, Groningen, NL | N/A | arm crank ergometer |
| Lode Ergometry Manager Software | Lode BV, Groningen, NL | N/A | Software |
| 10ul end-to-end capillary | EKF-diagnostics GmbH, Barleben, Germany | 0209-0100-005 | Capillaries |
| haemolysis cup | EKF-diagnostics GmbH, Barleben, Germany | 0209-0100-006 | hemolysis cup |
| lactate analyzer | Biosen C line, EKF-diagnostics GmbH | 5213-0051-6200 | lactate analyzer |
| Heart rate monitor, Polar 610i | Polar, Kempele, Finland | P610i | heart rate monitor |
| metabolic cart, Oxygen Pro | Jaeger GmbH | N/A | metabolic cart |
| oxygen mask, Hans Rudolph | Hans Rudolph Inc. , USA | 113814 | oxygen mask |
| statistical software, PSAW Software | SPSS Inc., Chicago USA | N/A | statistical software |
| desinfectant, Soft-Zellin | Hartmann GmbH, Austria | 999979 | desinfectant |
| Quality control cup, EasyCon Norm | EKF-diagnostics GmbH, Barleben, Germany | 0201-005.012P6 | quality control |
| Quality control cup 3mmol/L | EKF-diagnostics GmbH, Barleben, Germany | 5130-6152 | control cup |
| Chip sensor lactate analyzer | EKF-diagnostics GmbH, Barleben, Germany | 5206-3029 | chip sensor |
| Lactate system solution | EKF-diagnostics GmbH, Barleben, Germany | 0201-0002-025 | lactate system solution |
| lancet, Mediware Blutlanzetten | medilab | 54041 | lancet |
| Calibration gas, | Jaeger GmbH | 36-MC G020 | calibration gas |
| chair provided by distributor (ergoselect) | ergoline GmbH, Germany | N/A | chair provided by distributor |
References
- Conconi, F., Ferrari, M., Ziglio, P. G., Droghetti, P., Codeca, L. Determination of the anaerobic threshold by a noninvasive field test in runners. J Appl Physiol. 52 (4), 869-873 (1982).
- Strupler, M., Mueller, G., Perret, C. Heart rate-based lactate minimum test: a reproducible method. Br J Sports Med. 43 (6), 432-436 (2009).
- Black, M. I., Durant, J., Jones, A. M., Vanhatalo, A. Critical power derived from a 3-min all-out test predicts 16.1-km road time-trial performance. Eur J Sport Sci. 14 (3), 217-223 (2014).
- Burnley, M., Doust, J. H., Vanhatalo, A. A 3-min all-out test to determine peak oxygen uptake and the maximal steady state. Med Sci Sports Exerc. 38 (11), 1995-2003 (2006).
- Vanhatalo, A., Doust, J. H., Burnley, M. A 3-min all-out cycling test is sensitive to a change in critical power. Med Sci Sports Exerc. 40 (9), 1693-1699 (2008).
- Johnson, T. M., Sexton, P. J., Placek, A. M., Murray, S. R., Pettitt, R. W. Reliability analysis of the 3-min all-out exercise test for cycle ergometry. Med Sci Sports Exerc. 43 (12), 2375-2380 (2011).
- Cheng, C. F., Yang, Y. S., Lin, H. M., Lee, C. L., Wang, C. Y. Determination of critical power in trained rowers using a three-minute all-out rowing test. Eur J Appl Physiol. 112 (4), 1251-1260 (2012).
- Fukuda, D. H., et al. Characterization of the work-time relationship during cross-country ski ergometry. Physiol Meas. 35 (1), 31-43 (2014).
- Vanhatalo, A., McNaughton, L. R., Siegler, J., Jones, A. M. Effect of induced alkalosis on the power-duration relationship of "all-out" exercise. Med. Sci. Sports Exerc. 42 (3), 563-570 (2010).
- Jacobs, P. L., Johnson, B., Somarriba, G. A., Carter, A. B. Reliability of upper extremity anaerobic power assessment in persons with tetraplegia. J Spinal Cord Med. 28 (2), 109-113 (2005).
- Jacobs, P. L., Mahoney, E. T., Johnson, B. Reliability of arm Wingate Anaerobic Testing in persons with complete paraplegia. J Spinal Cord Med. 26 (2), 141-144 (2003).
- Flueck, J. L., Lienert, M., Schaufelberger, F., Perret, C. Reliability of a 3-min all-out arm crank ergometer exercise test. Int J Sports Med. 36 (10), 809-813 (2015).
- Erich Jaeger GmbH. . User Manual Oxycon Pro. , (2016).
- Shrout, P. E., Fleiss, J. L. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 86 (2), 420-428 (1979).
- Beckerman, H., et al. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res. 10 (7), 571-578 (2001).
- Plichta, S. B., Kelvin, E. A., Munro, B. H. . Munro's statistical methods for health care research. , (2011).
- Atkinson, G., Nevill, A. M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 26 (4), 217-238 (1998).
- Bland, J. M., Altman, D. G. Measuring agreement in method comparison studies. Stat Methods Med Res. 8 (2), 135-160 (1999).
- van Drongelen, S., Maas, J. C., Scheel-Sailer, A., Van Der Woude, L. H. Submaximal arm crank ergometry: Effects of crank axis positioning on mechanical efficiency, physiological strain and perceived discomfort. J. Med. Eng. Technol. 33 (2), 151-157 (2009).
- Bressel, E., Bressel, M., Marquez, M., Heise, G. D. The effect of handgrip position on upper extremity neuromuscular responses to arm cranking exercise. J. Electromyogr. Kinesiol. 11 (4), 291-298 (2001).
- West, C. R., Goosey-Tolfrey, V. L., Campbell, I. G., Romer, L. M. Effect of abdominal binding on respiratory mechanics during exercise in athletes with cervical spinal cord injury. J Appl Physiol (1985). 117 (1), 36-45 (2014).
- West, C. R., Campbell, I. G., Goosey-Tolfrey, V. L., Mason, B. S., Romer, L. M. Effects of abdominal binding on field-based exercise responses in Paralympic athletes with cervical spinal cord injury. J. Sci. Med. Sport. 17 (4), 351-355 (2014).
- Kirshblum, S. C., et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 34 (6), 535-546 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved