A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Easy Manipulation of Architectures in Protein-based Hydrogels for Cell Culture Applications
In This Article
Summary
Different methods to manipulate three-dimensional architecture in protein-based hydrogels are evaluated here with respect to material properties. The macroporous networks are functionalized with a cell-adhesive peptide, and their feasibility in cell culture is evaluated using two different model cell lines.
Abstract
Hydrogels are recognized as promising materials for cell culture applications due to their ability to provide highly hydrated cell environments. The field of 3D templates is rising due to the potential resemblance of those materials to the natural extracellular matrix. Protein-based hydrogels are particularly promising because they can easily be functionalized and can achieve defined structures with adjustable physicochemical properties. However, the production of macroporous 3D templates for cell culture applications using natural materials is often limited by their weaker mechanical properties compared to those of synthetic materials. Here, different methods were evaluated to produce macroporous bovine serum albumin (BSA)-based hydrogel systems, with adjustable pore sizes in the range of 10 to 70 µm in radius. Furthermore, a method to generate channels in this protein-based material that are several hundred microns long was established. The different methods to produce pores, as well as the influence of pore size on material properties such as swelling ratio, pH, temperature stability, and enzymatic degradation behavior, were analyzed. Pore sizes were investigated in the native, swollen state of the hydrogels using confocal laser scanning microscopy. The feasibility for cell culture applications was evaluated using a cell-adhesive RGD peptide modification of the protein system and two model cell lines: human breast cancer cells (A549) and adenocarcinomic human alveolar basal epithelial cells (MCF7).
Introduction
Hydrogels are materials that form insoluble 3D networks capable of binding large amounts of water. Such materials can provide excellent environmental conditions for living cells. Currently, there is increasing interest in the generation of three-dimensional hydrogel structures and in the development of processes to tailor their chemical and physical properties. Once this is achieved, a template for the growth of cells and the manipulation of cellular behavior can be generated1,2,3,4. These 3D structures not only create a more natural and realistic environment than conventional two-dimensional approaches, but they also reveal new possibilities for the growth of stem cells or tumor models5. Different materials possess a range of characteristics that mainly depend upon the pore size of the gel6. The pores play a crucial role in cell culture applications, tissue engineering, and the directed growth of stem cells. For example, oxygen and nutrients diffuse through the matrix, and adequate amounts must be able to reach the cells7. On the other hand, harmful metabolites must be removed as quickly as possible, and sufficient space for cell growth must be available7. Consequently, the properties of the material, and thus the pore size, severely influence the potential benefit and possible applications of the matrix. Depending upon the properties of the material, different cell-growth processes can occur in 3D cell culture, including the formation of neuronal structures; the growth and differentiation of skin or bone cells; and the directed growth of special stem cell lines, like hepatocytes or fibroblasts2,3,8,9,10,11. Another crucial point influencing the possible application of a material is its stability towards external stimuli12. For example, the hydrogel must maintain its mechanical integrity in cell culture media or the human body.
In recent years, research on 3D cell culture hydrogels intensified, and many studies were carried out to resolve the 3D architectures of the systems13. Hydrogels composed of chemically synthesized components are most commonly investigated because they can be easily synthesized and chemically modified and they exhibit high stability (see Zhu et al., 2011 for a review)5. However, proteins have many beneficial properties: as so-called "precision polymers," they are biocompatible; they have a defined length; they are relatively easy to modify; and they have a large number of target sites14,15. In this regard, highly specific, innovative structures can be generated for application in many fields. In this study, a protein-based hydrogel16 was used to demonstrate the ability of well-established methods to influence the 3D architecture of the material. Furthermore, the capability of and applicability to pore generation was also investigated.
Many different techniques are available to modify 3D structures, including both simple methods and sophisticated, highly specialized techniques from different fields of material science. A widespread technique is the use of electrospinning to generate well-defined structures17. Charged fibers are pulled from a solution by an electric field and then solidify upon exposure to oxygen. In this way, fibers in the range of several nanometers up to several microns can be produced. Additional techniques to tune the size, structure, and distribution of the pores within the matrix are soft lithography, photolithography, hydrodynamic focusing, electro-spraying, and bio-printing18,19,20. A significant drawback of these techniques is their dependency upon specific, expensive equipment and special chemicals or materials. Furthermore, experience with these techniques is often not directly transferrable to protein-based materials, and many of the chemicals and methods are not cell compatible.
On the other hand, many techniques do not rely upon special equipment, making them easier and cheaper to apply and to reproduce. A widespread method for structure manipulation is solvent casting21,22,23. Particles are added prior to the polymerization reaction and are distributed homogenously to saturate the solution. After the polymerization, a change of conditions, such as a dilution or a pH change, leads to the solvation of the particles, while the pores remain within the material. The chemicals used in these techniques, such as salt, sugar, paraffin, gelatin, and chalk, are cheap and readily available. In freeze-drying, swollen hydrogels are frozen. The subsequent sublimation of the liquid phases under a vacuum is then performed23,24,25. Water sublimation from the network is gentle enough to maintain the specific 3D structures of the material. In gas foaming, a solution is streamed with a gas while the polymerization takes place, leaving pores within the gel21. The size and distribution of the pores can be adjusted depending upon the gas stream.
To form the protein hydrogel, BSA is reacted with tetrakis (hydroxymethyl) phosphonium chloride (THPC) in a Mannich-type reaction to allow for the formation of covalent bonds between primary amines and the hydroxy groups of the four-armed linker molecule26. Possible harmful intermediates are removed by excessive washing of the material after the reaction occurs.
This study demonstrates the possibility of treating a BSA-based material with different techniques to manipulate and tailor the size of the pores. Each of the techniques can be used in any laboratory worldwide, as no special equipment is necessary. In addition, different parameters, such as swelling ratio, enzymatic degradability, pH stability, and temperature sensitivity, were examined and compared to each other, especially respect to the influence of the different techniques on the generation of 3D architectures. Finally, the materials were functionalized with cell-adhesive peptides to investigate the possible application of the materials to cell culture. Two different model cell lines were used: A549 and MCF7.
Protocol
1. Hydrogel Preparation
- Mix 200 mg of BSA with 1 mL of deionized H2O to create 20% (w/v) BSA stock (stock solution A).
- Mix 165 µL of THPC solution (134 mg/mL) with 4.835 mL of deionized water to create THPC stock solution (stock solution B).
- Weigh 1 mg of KCSSGKSRGDS (1,111.1 g/mol) peptide (or an equivalent cell-adhesive peptide) and dilute it in 100 µL of sterile H2O to obtain a 10 mg/mL solution (stock solution C).
NOTE: This step is optional and only needs to be included if the hydrogel is meant for cell culture application. - Remove the bottom of a 96-well plate and replace it with removable plastic wrap.
NOTE: For the plates used here, the bottom can easily be removed by applying pressure to the bottom of each well; the thin plastic bottom will simply fall out. - Mix 100 µL of BSA stock solution (A) and 100 µL of THPC stock solution (B) (optional: 4 µL of stock solution C for functionalized, cell-adhesive hydrogels) in the 96-well plate to obtain 200 µL of hydrogel. Mix the components by pipetting up and down at least 5 times to guarantee a uniform hydrogel after polymerization.
- Place the 96-well plate at room temperature (RT) for about 10 min until all hydrogels are properly polymerized.
- Carefully and slowly remove the plastic wrap from the bottom of the plate.
- Press the hydrogels out of the 96-well plate using a small stamp and transfer them to 1.5 mL tubes with sterile PBS, pH 7.4.
NOTE: The hydrogels have a cylindrical shape, with a diameter of approximately 4 mm and a height of 8 mm. - Store the hydrogels in phosphate-buffered saline (PBS) at 4 °C for up to several months.
2. Freeze-drying the Hydrogels
- Fill 1.5 mL tubes with 500 µL of sterile deionized H2O and remove the cap. Transfer the hydrogels to the 1.5 mL reaction tubes using a spatula.
- Wrap the 1.5 mL tubes tightly with paraffin film-at least three layers for each vial. Use a needle to pierce small holes in the film to enable gas release from the tube.
- Proceed to one of the following steps:
- Transfer the vial to liquid nitrogen solution for 5 min to guarantee that the water and hydrogel completely freeze. Immediately after removal from the liquid nitrogen, transfer the vials to the freeze dryer to prevent the material from thawing.
NOTE: This procedure results in pores about 10-15 µm in radius. - Keep the vials at -20 °C overnight to slowly freeze the hydrogels. Immediately after removal from -20 °C, transfer the vials to the freeze dryer to prevent the material from thawing.
NOTE: This procedure results in pores about 50-60 µm in radius.
- Transfer the vial to liquid nitrogen solution for 5 min to guarantee that the water and hydrogel completely freeze. Immediately after removal from the liquid nitrogen, transfer the vials to the freeze dryer to prevent the material from thawing.
- After 24 h (and the complete evaporation of the water in and around the hydrogel), thaw the material by removing it from the freeze dryer.
NOTE: The pore sizes can be analyzed with confocal laser scanning microscopy (step 5, "Hydrogel Visualization").
3. Particle Leaching
- Prepare the hydrogel as described in steps 1.1-1.5.
- Directly after mixing the components, add NaCl until saturation occurs (36 mg/mL). Add salt until a salt crystal can be seen in the solution as a white precipitate.
- Transfer the 96-well plate to a shaker and shake until polymerization takes place (about 10 min). Remove the hydrogels from the plate, as described in steps 1.7-1.8.
- Incubate the hydrogels for at least 24 h at RT in sterile water on a shaker (20 rpm) to elute all salt from the hydrogel template. Store the hydrogels at 4 °C in PBS for up to several months.
4. Channel Formation
- Prepare hydrogels as described in step 1. Remove a hydrogel from solution using a pincer, remove the excess water with highly absorbent paper, and place it on top of a block of dry ice.
- Freeze the hydrogel for 30 s and carefully remove it from the block. Do not damage the hydrogel; carefully use a spatula to scrape it off the block. Transfer the hydrogel to a 1.5 mL tube and dry it overnight at 37 °C.
5. Hydrogel Visualization
- Prepare a rhodamine B stock solution by diluting 1 mg of rhodamine B in 10 mL of PBS. Prepare a serial dilution by diluting the rhodamine stock solution in PBS until a concentration of 0.001 mg/mL is reached (dilution factor: 100)
- Remove the hydrogel from the storage solution (e.g., from step 2.4.) and transfer it to a 1.5 mL tube with 1 mL of 0.01 mg/mL rhodamine B solution. Stain the hydrogel overnight in rhodamine B solution at RT.
- The next day, transfer the hydrogel that is to be visualized to 10 mL of PBS (pH 7.4) and wash for at least 3 h.
- Transfer the hydrogel onto a µ-slide 8 well and cover it with PBS.
- Cut small slices out of the hydrogel (about 0.5 mm in height) using a blade.
- Using a confocal laser scanning microscope, visualize the hydrogel at a wavelength of 514 nm (objective: EC Plan-Neofluar 40x/1.30 Oil DIC M27, plane scan mode: 514 nm, and zoom: 1X).
6. Cell Culture Feasibility
- Sterile-filter all stock solutions (A, B, and C) with a 0.45 µm filter.
- Prepare the hydrogel as described in steps 1.1-1.4, including the cell-adhesive peptide prior to hydrogel polymerization.
- After mixing the components, immediately pipette the mixture into a µ-slide 8 well until the bottom is completely covered.
NOTE: This step must be performed quickly and directly after mixing the components, as polymerization takes place within minutes in the slide. - Culture adhesion cells and passage to obtain a single-cell suspension using standard cell culture techniques. Transfer the cells into pre-warmed, sterile DMEM cell culture medium supplemented with fetal bovine serum (FBS, 10% (w/v)), penicillin-streptomycin (1% (w/v)), and nonessential amino acid solution (MEM, 1% (w/v)).
- Count the cells with a Neubauer counting chamber and carefully pipette 200 µL of the desired number of cells (2 x 105 cells/cm2) onto the hydrogel surface.
- Cover the µ-slide 8 well with the lid and transfer it to an incubator (37 °C, 5% CO2). Incubate for at least 4 h at 37 °C.
- After at least 4 h of cellular attachment, wash the cells twice with 200 µL of sterile cell culture PBS.
- Fix the cells with 200 µL of 3.7% (v/v) formaldehyde for 10 min at RT and wash twice with PBS (~200 µL).
NOTE: Wear appropriate personal protective equipment when handling formaldehyde. - Permeabilize the cells with 200 µL of 0.1 % Triton X for 5 min. Wash twice with PBS.
- Stain the cells with phalloidin-rhodamine by mixing 5 µL of methanolic stock with 195 µL of PBS and adding it to the cells at RT. Stain the cells for 20 min in the dark. Wash twice with PBS.
- Investigate the cell adhesion properties using a confocal microscope at 514 nm (objective: EC Plan-Neofluar 40x/1.30 Oil DIC M27, plane scan mode: 514 nm, and zoom: 1X). Analyze the images using appropriate software (see the Table of Materials).
7. Hydrogel Properties
- Swelling ratio.
- Completely dry the hydrogels at 37 °C for at least one day.
- Weigh each hydrogel and note the exact weight.
- Fill a 2 mL reaction tube with 1.5 mL of PBS.
- Transfer the hydrogel into this 2 mL reaction tube and completely immerse it in PBS.
- Leave the hydrogel for at least two days in PBS at RT to reach equilibrium with the PBS.
- After three days, remove the hydrogel from the solution and dry it with a paper tissue to remove excess water from the hydrogel surface.
- Weigh the hydrogel.
- Calculate the swelling ratio using the following formula:
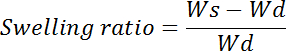
where Wd is the weight of the dried gel (step 7.1.2.) and Ws is the weight of the wet gel (step 7.1.7). Multiply with 100 to get the swelling ratio percent.
- pH and temperature stability.
- Transfer 5 mL of PBS to a 15-mL reaction tube.
- Adjust the solution to the appropriate pH (e.g., pH 2, 7, or 10) with NaOH and HCl. Bring the solution to the appropriate temperature (e.g., RT, 37 °C, or 80 °C).
- Transfer the hydrogel that is to be investigated for its stability into the appropriate solution. Readjust the pH if necessary.
NOTE: All hydrogels should be completely swollen at this point (see the swelling ratio) to prevent the incorrect interpretation of the weight due to the swelling of the material. - After certain time intervals, remove the hydrogel from the solution (e.g., each hour for up to 2 days), dry it with a highly absorbent paper tissue to remove excess water, and weigh it.
- Enzymatic degradation.
- Prepare a stock enzyme solution of 300 U trypsin and pepsin, as per the manufacturer's instructions.
- Transfer the hydrogel (e.g., from step 1.8) to the appropriate solution.
NOTE: All hydrogels should be completely swollen at this point to prevent the incorrect interpretation of the weight. - After certain time intervals, remove the hydrogel from the solution (e.g., each hour), dry it with a highly absorbent paper tissue to remove excess water, and weigh it.
Results
Hydrogel development has become one of the most prominent fields in material research-related biological studies, with thousands of entries indexed in scientific research archives. Although the behavior of many systems is well studied, the manipulation of 3D networks, especially of sensitive protein-based materials, is often a major issue in material science. Another commonly underestimated challenge is the correct measurement of the native structure of a material using cryo electron micr...
Discussion
The production of macroporous matrices can be beneficial to many different fields. It has high technical and economic potential due to the defined structure of the hydrogel and the ability to control and tune specific material properties. However, the introduction of supramolecular structural elements, such as pores or channels, to a 3D template might influence the overall properties of a material, such as the swelling ratio or the stiffness. This can result in the undesired decomposition, degradation, or breakdown of th...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
The authors would like to thank Baden-Württemberg Stiftung for their financial support in the "Bioinspired Material Synthesis" framework (BioMatS-14).
Materials
| Name | Company | Catalog Number | Comments |
| Phosphate Buffered Saline (PBS) | Thermo Fisher Scientific | 10010023 | |
| Dulbecco’s modified Eagle’s medium (high glucose) | Life Technologies / Thermo Fisher | 11140-050 | |
| Fetal Bovine Serum (FBS) | Life Technologies / Thermo Fisher | 10270-106 | |
| Penicillin-Streptomycin | Life Technologies / Thermo Fisher | 15140122 | |
| MEM Nonessential Amino Acid Solution | Sigma Aldrich | M7145-100ML | |
| Trypsin EDTA 0.05% Phenol Red | Thermo Fisher Scientific | 25300062 | |
| Ethanol 99.8%, vergällt | Ölfabrik Schmidt | 2133 | |
| NaCl | Carl Roth | 9265.1 | |
| Albumin Fraction V | Carl Roth | 3854.2 | |
| THPC | Sigma Aldrich | 404861-100ML | Toxic |
| 0.1% Triton X-100 | Sigma Aldrich | X100-100ML | Slightly toxic |
| Phalloidin-rhodamine | Life Technologies / Thermo Fisher | R415 | |
| 3.7% Formaldehyde | Life Technologies / Thermo Fisher | F8775-25ML | Toxic |
| Rhodamine B | Sigma Aldrich | 81-88-9 | |
| Filtropur S 0.2 | Sarsted Ag und Co. | 2 83.1826.001 | |
| µ slide 8 well | Ibidi GmbH | 80826 | |
| KCSSGKSRGDS peptide | UPEP Ulm | Custom sysnthesis | |
| Ethanol 99.8%, vergällt | Carl Roth | K928.5 | |
| Falcon 5 ml Polysterene Round-Bottom Tube | Sarsted Ag und Co. | 62.547.254 | |
| Tubes 50 ml | Sarsted Ag und Co. | 62.547.254 | |
| Tubes 1.5 ml | Sarsted Ag und Co. | 72,690,001 | |
| Tubes 2 ml | Sarsted Ag und Co. | 72,691 | |
| CELL CULTURE MICROPLATE, 96 WELL, PS, F-BOTTOM | Greiner | 655073 | |
| FreezeDryer Epsilon 1-6D, | Christ, Osterode am Harz, Germany | ||
| Confocal Laser Scanning Microscope | Carl Zeiss AG, Oberkochen, Germany | ||
| Zen Software Version 2012 Sp1, black edition, 407 version 8,1,0,484 | Carl Zeiss AG, Oberkochen, Germany | ||
| GSA Imaga Analyzer Software, GSA Image Analyzer, GSA, Version 419 3.8.7 | GSA GmbH |
References
- Geckil, H., Xu, F., Zhang, X., Moon, S., Demirki, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 5 (3), 469-484 (2011).
- Liu, Y., Chan-Park, M. B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials. 31 (6), 1158-1170 (2010).
- Raic, A., Rödling, L., Kalbacher, H., Lee-Thedieck, C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 35 (3), 929-940 (2014).
- Wong Po Foo, C. T. S., Lee, J. S., Mulyasasmita, W., Parisi-Amon, A., Heilshorn, S. C. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc Nat Acad Sci USA. 106 (52), 22067-22072 (2009).
- Zhu, J., Marchant, R. E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devic. 8 (5), 607-626 (2011).
- Samaryk, V., et al. Versatile Approach to Develop Porous Hydrogels with a Regular Pore Distribution and Investigation of their Physicomechanical Properties. J Appl Polym Sci. 114 (4), 2204-2212 (2009).
- Li, X. J., Valadez, A. V., Zuo, P., Nie, Z. Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis. 4 (12), 1509-1525 (2012).
- Park, H., Guo, X., et al. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules. 10 (3), 541-546 (2010).
- Stokols, S., Tuszynski, M. H. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 25 (27), 5839-5846 (2004).
- Sung, K. E., et al. Understanding the Impact of 2D and 3D Fibroblast Cultures on In Vitro Breast Cancer Models. PLoS One. 8 (10), 1-13 (2013).
- Tsai, E. C., Dalton, P. D., Shoichet, M. S., Tator, C. H. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 21 (6), 789-804 (2004).
- Shoichet, M. S., Li, R. H., White, M. L., Winn, S. R. Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose. Biotechnol Bioeng. 50 (4), 374-381 (1996).
- Chiu, Y. -. C., Kocagöz, S., Larson, J. C., Brey, E. M. Evaluation of physical and mechanical properties of porous poly (ethylene glycol)-co-(L-lactic acid) hydrogels during degradation. PloS One. 8 (4), e60728 (2013).
- Jonker, A. M., Löwik, D. W. P. M., van Hest, J. C. M. Peptide- and Protein-Based Hydrogels. Chem Mater. 24 (5), 759-766 (2012).
- Bodenberger, N., et al. Beyond bread and beer: whole cell protein extracts from baker's yeast as a bulk source for 3D cell culture matrices. Appl Microbiol Biot. 101 (5), 1-11 (2016).
- Bodenberger, N., Paul, P., Kubiczek, D., Walther, P., Gottschalk, K. E., Rosenau, F. A novel cheap and easy to handle protein hydrogel for 3D cell culture applications a high stability matrix with tunable elasticity and cell adhesion properties. Chem Sel. 1 (7), 1353-1360 (2016).
- Agarwal, S., Wendorff, J. H., Greiner, A. Use of electrospinning technique for biomedical applications. Polymer. 49 (26), 5603-5621 (2008).
- Hong, J., deMello, A. J., Jayasinghe, S. N. Bio-electrospraying and droplet-based microfluidics: control of cell numbers within living residues. Biome Mater. 5 (2), 21001 (2010).
- Jayasinghe, S. N., Irvine, S., McEwan, J. R. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine. 2 (4), 555-567 (2007).
- Selimović, &. #. 3. 5. 2. ;., Oh, J., Bae, H., Dokmeci, M., Khademhosseini, A. Microscale strategies for generating cell-encapsulating hydrogels. Polymers. 4 (3), 1554-1579 (2012).
- Annabi, N., Nichol, J. W., et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 16 (4), 371-383 (2010).
- Lee, J., Cuddihy, M. J., Kotov, N. A. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 14 (1), 61-86 (2008).
- Bodenberger, N., et al. Evaluation of methods for pore generation and their influence on physio-chemical properties of a protein based hydroge. Biotech Rep. 12, 6-12 (2016).
- Raja, S. T. K., Thiruselvi, T., Mandal, A. B., Gnanamani, A. pH and redox sensitive albumin hydrogel: A self-derived biomaterial. Sci Rep. 5, 15977 (2015).
- Hennink, W. E., van Nostrum, C. F. Novel crosslinking methods to design hydrogels. Adv Drug Deliver Rev. 64, 223-236 (2012).
- Chung, C., Lampe, K. J., Heilshorn, S. C. Tetrakis(hydroxymethyl) phosphonium chloride as a covalent cross-linking agent for cell encapsulation within protein-based hydrogels. Biomacromolecules. 13 (12), 3912-3916 (2008).
- Caló, E., Khutoryanskiy, V. V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 65, 252-267 (2015).
- Huang, H., Herrera, A. I., Luo, Z., Prakash, O., Sun, X. S. Structural transformation and physical properties of a hydrogel-forming peptide studied by NMR, transmission electron microscopy, and dynamic rheometer. Biophys J. 103 (5), 979-988 (2012).
- Draghi, L., Resta, S., Pirozzolo, M. G., Tanzi, M. C. Microspheres leaching for scaffold porosity control. J Mater Sci. 16 (12), 1093-1097 (2005).
- Whang, K., et al. Engineering Bone Regeneration with Bioabsorbable Scaffolds with Novel Microarchitecture. Tissue Eng. 5 (1), 35-51 (1999).
- Ziv, K., et al. A tunable silk-alginate hydrogel scaffold for stem cell culture and transplantation. Biomaterials. 35 (12), 3736-3743 (2014).
- Butruk-Raszeja, B. A., et al. Athrombogenic hydrogel coatings for medical devices--Examination of biological properties. Colloid Surface B. 130, 192-198 (2015).
- Lü, S., Li, B., Ni, B., Sun, Z., Liu, M., Wang, Q. Thermoresponsive injectable hydrogel for three-dimensional cell culture: chondroitin sulfate bioconjugated with poly(N-isopropylacrylamide) synthesized by RAFT polymerization. Soft Matter. 7 (22), 10763 (2011).
- Ruoslahti, E., Pierschbacher, M. D. New perspectives in cell adhesion: RGD and integrins. Science. 238 (4826), 491-497 (1987).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved