A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
High Fat Diet Feeding and High Throughput Triacylglyceride Assay in Drosophila Melanogaster
In This Article
Summary
This is a high fat diet feeding protocol to induce obesity in Drosophila, a model for understanding fundamental molecular mechanisms implicated in lipotoxicity. It also provides a high throughput triacylglyceride assay for measuring fat accumulation in Drosophila and potentially other (insect) models under various dietary, environmental, genetic or physiological conditions.
Abstract
Heart disease is the number one cause of human death worldwide. Numerous studies have shown strong connections between obesity and cardiac malfunction in humans, but more tools and research efforts are needed to better elucidate the mechanisms involved. For over a century, the genetically highly tractable model of Drosophila has been instrumental in the discovery of key genes and molecular pathways that proved to be highly conserved across species. Many biological processes and disease mechanisms are functionally conserved in the fly, such as development (e.g., body plan, heart), cancer, and neurodegenerative disease. Recently, the study of obesity and secondary pathologies, such as heart disease in model organisms, has played a highly critical role in the identification of key regulators involved in metabolic syndrome in humans.
Here, we propose to use this model organism as an efficient tool to induce obesity, i.e., excessive fat accumulation, and develop an efficient protocol to monitor fat content in the form of TAGs accumulation. In addition to the highly conserved, but less complex genome, the fly also has a short lifespan for rapid experimentation, combined with cost-effectiveness. This paper provides a detailed protocol for High Fat Diet (HFD) feeding in Drosophila to induce obesity and a high throughput triacylglyceride (TAG) assay for measuring the associated increase in fat content, with the aim to be highly reproducible and efficient for large-scale genetic or chemical screening. These protocols offer new opportunities to efficiently investigate regulatory mechanisms involved in obesity, as well as provide a standardized platform for drug discovery research for rapid testing of the effect of drug candidates on the development or prevention of obesity, diabetes and related metabolic diseases.
Introduction
We are in a time where obesity, and its associated economic burdens, is a worldwide problem1. Two out of every three Americans are overweight or obese with related heart pathologies, the primary cause of death within the adult population2. New efficient methods are needed to adequately investigate the genetic and molecular components implicated in the regulation of metabolic syndrome using model organisms. For this reason, we choose the fruit fly Drosophila model because it shares the most basic biological processes with mammals, including mice and humans3,4,5,6. Drosophila's genome is highly conserved during evolution but overall much smaller with less gene duplication and metabolic complexity, making it ideal for understanding the fundamental mechanisms implicated in many human diseases4,7,8. Also, characteristic processes carried out by adipose tissue, the gut and pancreas are represented in the fly and mediate regulatory functions in glucose and lipid metabolism, for example, that are similar to humans9,10,11. Moreover, the basic molecular pathways involved in the control of obesity, insulin resistance and diabetes in humans are functionally conserved in Drosophila melanogaster12,13,14,15,16. Like higher organisms, Drosophila has a beating heart that is formed during development by similar processes to that of the mammalian heart3,17. Thus, the development of a reliable HFD feeding protocol and high throughput TAG assay, adapted for efficient screening purposes using the genetic tool box of Drosophila, provide an important means to study and understand the fundamental genetic basis underlying complex metabolic diseases.
The HFD food itself is made from a standard laboratory fly food supplemented with coconut oil, which is composed mostly of saturated fatty acids known to be associated with metabolic syndrome18. While inducing obesity in mammalian models, such as rodents, can take months19,20, our optimized HFD feeding protocol in Drosophila effectively and reproducibly increases organismal fat content in a matter of days12,14. This protocol, in conjunction with a high throughput TAG assay, allows efficient mass screening for the effects of genetic factors, environmental influences and drug candidates to discover new modulators of fat metabolism. In consequence, these protocols are likely relevant to understand and/or combat obesity and obesity-associated human pathologies.
The feeding protocol is versatile and may be applied to study the metabolic and functional effects of single saturated or unsaturated fatty acid. The use of this high throughput TAG assay is not limited to D. melanogaster, but may be adapted to a variety of small model organisms with cuticle or tough extracellular matrices (e.g., other Drosophila species, C. elegans and other emerging invertebrate model organisms) to measure fat content under different environmental, genetic or physiological conditions, at any stage of development, adulthood or phase of metabolic disease. The TAG assay is based on a colorimetric measurement of a series of enzymatic reactions that degrade the TAGs into free fatty acids, glycerol, Glycerol 3-phosphate and finally H2O2 that reacts with 4-aminoantipyrine (4-AAP) and 3,5-dichloro-2-hydroxybenzene sulfonate (3,5 DHBS) to produce a red colored product that is measured using a 96-well spectrophotometer.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. HFD Feeding Protocol
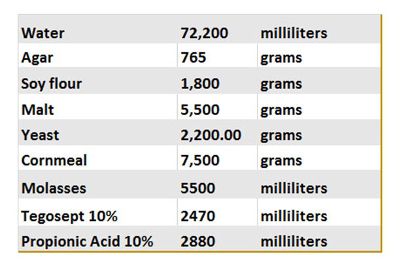
Table 1. Fly food recipe.
This table summarizes the different ingredients used to prepare our control food. Once made, 10 mL of the food is poured into vials, cooled and stored at 4 °C for long term storage.
- HFD preparation
- To make 1 kg of high fat food, weigh 700 g of pre-made normal fly food (see Table 1) and 300 g of coconut oil into two separate containers using an analytical balance.
- Heat each container individually in the microwave, until the contents melt completely. Pour the coconut oil into the fly food container. Stir using a whisk until the oil is well mixed into the fly food.
NOTE: No chunks of food or oil should be visible. When the fly food is melting in the microwave, stop at intervals of 1 min to mix the food and prevent boiling over. - Re-weigh the high fat food. If the total weight is less than 1 kg (700 g of food + 300 g of coconut oil), add water (around 10 mL) to compensate for evaporation during heating and to bring back the weight to 1 kg.
- Pour about 10 mL well-mixed high fat food per vial (around 25% of vial volume). Next, cover with cheesecloth to prevent flies' contamination. Cool for 1 h at room temperature then transfer the vials to 4 °C for storage up to 4 weeks.
NOTE: The Normal food (NF) is used as a control food. Also pour 10 mL (around 25% of vial volume) of NF per vial. For the HFD food, replace 30% of the NF food with coconut oil. For control and experimental conditions, the flies were given the same amount of NF and HFD (10 mL of food per vial).
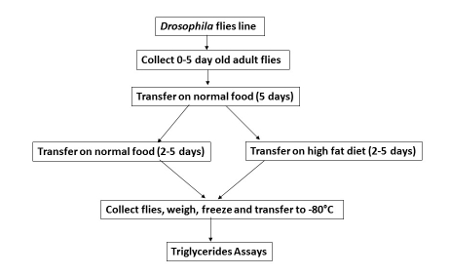
Figure 1. HFD feeding in Drosophila.
The scheme shows the different feeding steps for flies on a control food (normal diet without addition of coconut oil-NF) or HFD (with the addition of coconut oil). The entire process takes 10 days after the initial collection of adult flies. Please click here to view a larger version of this figure.
- HFD feeding of D. melanogaster
NOTE: This protocol includes control food and HFD feeding procedures that are compatible with any fly line. Control and experimental flies are put in vials containing the same amount food (NF or HFD).- Use CO2 to anesthetize the flies.
NOTE: For neophytes, please read reference21 for an overview on raising and handling D. melanogaster. - Collect 0-5 day old flies from vials and transfer (by flipping) the flies into new vials containing NF (previously warmed at room temperature). Let the flies age for 5 additional days at 21 °C.
- At the end of the 5 days, take out the vials containing HFD and NF from 4 °C.
NOTE: As cold temperatures can stress living flies, let the vials sit for 30 min at room temperature to warm up before use. - Using wipes, clean and remove excess fluid from the sides of the vials.
- Insert a small piece of filter paper, about 1 cm x 3 cm, into the high fat food to absorb any surplus liquid.
NOTE: This step is critical for preventing the flies from sticking to the HFD food. - Next, transfer the flies onto the high fat food (by flipping). Lay the vials horizontally on their side, (not standing) at 21-22 °C for 5 days.
NOTE: Avoid higher temperatures as this can partially melt the high fat food, causing the flies to stick to the food and die. - After 5 days, transfer (by flipping) the flies from NF and HFD into new vials without food to allow their guts to empty for 30 min. Next, proceed to weighing the flies.
NOTE: One of the critical steps in this HFD feeding protocol is to prepare a well-mixed HFD, free of any chunks of fly food or condensed oil. Homogenous mixing and cooling the HFD to 45-30 °C is advisable, before pouring into vials and storing the food at 4 °C. Proper age matching of control and experimental flies is important as well as controlled nutritional and environmental conditions prior to the HFD feeding. During the feeding phase, control and experimental flies must be given the same amount of NF and HFD. Never collect flies from overcrowded vials or bottles (to avoid transgenerational effects). Always put a maximum of 25 flies per vial during NF and HFD feeding to avoid dietary restriction or other crowding effects. The HFD is composed of 30% coconut oil, thus temperatures above 22 °C might partially melt the HFD, causing the flies to stick on the oily food and die. Before putting the flies on the HFD, clean the vials to remove any residual oil and insert a filter paper to absorb any excess fluid in the food. During the incubation of the flies on HFD, the vials are laid on their sides to provide a fat-free horizontal surface for the flies.
- Use CO2 to anesthetize the flies.
- Weighing the flies
- For each genotype and gender to be tested, use an ultra-sensitive scale to record the weights of pre-labeled, empty 1.7 mL tubes.
- Using fly anesthetic or CO2, anesthetize and collect 36 flies of the same genotype, gender, and feeding status with a brush into one pre-weighed 1.7 mL tube.
- Weigh the tube containing flies with the same ultra-sensitive scale.
- Determine the average fly weight following this formula:
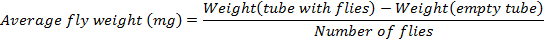
NOTE: The number of flies in this protocol is 36. - Flash freeze the 1.7 mL tubes containing flies by plunging into liquid nitrogen for 2 min. Collect and put the tubes into boxes. Next, transfer the boxes to -80 °C (preferable temperature) for storage until use with the TAG assay.
CAUTION: Liquid nitrogen is at an extremely low temperature. Please use appropriate personal protective gear.
2. TAG Assay
- Preparation of the TAG standard concentrations
- Prepare 500 mL of PBT (PBS 1X, 0.05% Triton).
- Using 0.5 mL tubes and the TAG solution (Table of Materials), prepare 100 µL of blank (PBT only) and 100 µL of six standard TAG concentrations (2 µg/µL; 1 µg/µL; 0.5 µg/µL; 0.25 µg/µL; 0.125 µg/µL; 0.0625 µg/µL) with the PBT.
- Place the tubes on ice and proceed with grinding the flies.
- Grinding the flies
- Take out the frozen flies from -80 °C (step 1.3). Transfer the tubes containing the flies on ice.
- First, place 2 mm metal grinding balls into a 96-well ball dispenser, use a small paintbrush to remove extra balls.
- Place the 96-well grinding plate upside down on top of the ball dispenser and flip to transfer the metal balls directly into the grinding plate. There should be one ball per well.
- Using a multi-channel pipette, add 600 µL of PBT into each row of the 96-well grinding plate.
- With forceps, add 3 flies per well. Indicate the genotype, gender and food condition of the flies in each row/well of the 96-well grinding plate.
- Tightly place the caps onto the 96-well grinding plate. Tape may be placed on top to ensure that there is no leakage.
- Properly place the 96-well grinding plates into the grinding machine. Set the machine to the highest speed and press "run" to grind the flies for 3 min.
- After 3 min, stop the grinding and proceed with plate centrifugation: centrifuge for 15 min at 4,000 x g, 4 °C. After centrifugation, manage the grinding plate carefully to avoid mixing the supernatant with the pellet.
- Sample loading and TAG content determination
- Take a new 96-well spectrophotometer plate. Using a multi-channel pipette, load 200 µL of the TAG reagent into each row of the plate.
- Load 20 µL of PBT into the first well of the first row, to create a blank. Mix by pipetting up and down. Avoid forming bubbles.
- Load 20 µL of each standard dilution (from step 2.1.2) into the remaining wells of the first row and mix by pipetting up and down. Avoid forming bubbles.
- Using a multi-channel pipette, transfer 20 µL of supernatant from each row of the grinding plate to the corresponding row in the 96-well spectrophotometer plate. Pipette up and down to mix well. Avoid forming bubbles.
- Next, place a gas-permeable foil over the top of the 96-well plate.
- Incubate the plate at 37 °C for 10 min.
NOTE: If there are bubbles in the wells, centrifuge the plate for 2 min at 2,000 x g to remove all bubbles. The presence of bubbles will interfere with the absorbance reading. - Read the absorbance of each well of the plate at 550 nm using a compatible microplate reader. Next, trace the standard curve and determine the concentration [µg/µL] of the unknown samples.
- To determine the amount of TAG content per mean fly weight, use the following formula:
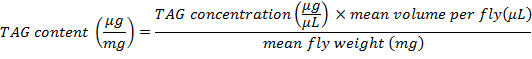
NOTE: In this protocol, 3 flies were minced in 600 µL of PBT; in consequence, the mean volume per fly is 200 µL.
- Bradford assay and normalization of TAG content with protein content
- Prepare seven 100 µL of bovine serum albumin (BSA) standard dilutions (75 µg/mL; 100 µg/mL; 150 µg/mL; 200 µg/mL; 250 µg/mL; 500 µg/mL; 1,000 µg/mL) with the PBT.
- Take a new 96-well plate and load 200 µL of 1X Bradford reagent into each row of the plate.
- In the first well of the first row, add 10 µL of PBT to create a blank. Mix by pipetting up and down. Avoid forming bubbles.
- Add 10 µL of each standard dilution into the remaining wells of the first row and mix by pipetting up and down. Avoid forming bubbles.
- Using a multi-channel pipette, transfer 10 µL of the fly supernatant from each row of the grinding plate into the corresponding row in the 96-well plate. Pipette up and down to mix well. Avoid forming bubbles.
- Place a gas-permeable foil over the top of the 96-well plate.
- Incubate the plate at room temperature for 5 min. If there are bubbles in the wells, centrifuge the plate for 2 min at 2,000 x g to remove them.
NOTE: The presence of bubbles will interfere with the absorbance reading. - Use a spectrophotometer to read the absorbance of the blank, standards and unknown samples at 595 nm. Use the standard curve to determine the concentrations [µg/µL] of the samples in each row.
- Normalize the TAG content with protein level using the following formula:

Access restricted. Please log in or start a trial to view this content.
Results
In D. melanogaster, as is the case with other species, there is sexual dimorphism between males and females22. It is well known that females are larger, with more fat in their abdomens, than males22. To test the effectiveness of our protocol, we performed TAG assays to determine the differences in TAG content between males and females of standard laboratory wildtype (w1118) flies. The data show that females have ...
Access restricted. Please log in or start a trial to view this content.
Discussion
Obesity induction in mice can take months19,20. In flies, this HFD feeding protocol allows for induction of excess fat accumulation in a matter of days or less, causing increases in fat accumulation only after 18 h (see Figure 2). HFD feeding with the described protocol increases glucose content 12 and decreases Bmm lipase and PGC-1 expression24. This is in contrast to fasting ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Erika Taylor for editing this manuscript. This work was funded by grants from the National Institutes of Health (P01 HL098053, P01 AG033561 and R01 HL054732) to R.B., a post-doctoral research supplement (R01 HL085481) and fellowship (AAUW) to S.B.D., and grants from the American Heart Association to S.B.D. and R.T.B.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Talboys Ball dropper/bead Dispenser | Talboys | #: 930150 | |
| Talboys High Throughput Homogenizer | Talboys | #: 930145 | |
| Grinding Balls, Stainless Steel | OPS Diagnostics, LLC | # GBSS 156-5000-01 | 5000 balls |
| Masterblock 96 Well deep Microplates | Greiner Bio-One | # T-3058-1 | case of 80 plates |
| Greiner 96 well microplate flat bottom | Sigma Aldrich | # M4436 | 40 plates |
| Greiner CapMat for sealing multiwell plates | Sigma Aldrich | # C3606 | 50 sealing plates |
| Reagent Reservoirs | Thomas Scientific | # 1192T71 | 12/PK |
| Thermo Scientific Finnpipette 4661040 | Thermo Scientific | # 4661040 | 1-10 ul multipipette |
| Thermo Scientific Finnpipette 4661070 | Thermo Scientific | # 4661070 | 30-300ul multipipette |
| Thermo Scientific Finnpipette 4661020 | Thermo Scientific | #4661020 | 10-100ul multipipette |
| Multichannel tips | Denville Scientific Inc | # P3131-S | for 10 uL pipette |
| Multichannel tips | Denville Scientific Inc | # P3133-S | for 200 uL pipette |
| Multichannel tips | Denville Scientific Inc | #P1125 | for 100 uL pipette |
| Forceps | Roboz Surgical | # 5 Dumonts | Super fine forceps |
| Mettler Toledo Excellence XS Analytical Balance Mfr# XS64 | Cole-Parmer scientific experts | # EW-11333-00 | |
| Metler Toledo Excellence XS Toploading Balance | Cole-Parmer scientific experts | # EW-11333-49 | |
| 96-Well microplate Centrifuge | Hettich Zentrifugen | # Rotina 420R | |
| Microplate Reader | Molecular devices | # SpectraMax 190 | |
| Lab-Line Bench Top Orbit Environ Shaker Incubator | Biostad | # 3527 | |
| Infinity Triglycerides reagent | Thermo Scientific | # TR22421 | |
| Triglyceride Standard | Stanbio | #2103 - 030 | |
| Quick Start Bradford Protein Assay | Bio-RAD | # 500-0205 | 1x dye Reagent |
| Coconut oil | Nutiva | # 692752200014 | 15 0z jar |
| PBS 10X | Thermo Scientific | # AM9625 | 500 ml |
| Triton X-100 | Sigma Aldrich | # 9002-93-1 | |
| Gas-permeable Foil | Macherey-Nagel | # 740675 | 50 pieces |
| filter Paper | VWR | # 28317-241 | Pack of 100 |
| Drosophila vials | Genesee Scientific | Cat #: 32-116SB | |
| Quick Start Bovine Serum Albumin Standard | Bio-Rad | # 5000206 | |
| FlyNap Anesthetic | Carolina | # 173025 | 100 mL |
| Kimwipes Low-Lint | Uline | # S-8115 | 1-Ply, 4.4 x 8.4" |
References
- Ng, M., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 384, 766-781 (2014).
- Murphy, S. L., et al. Mortality in the United States, 2014. NCHS data brief, no 229. , Available from: https://www.cdc.gov/nchs/products/databriefs/db229.htm (2015).
- Bodmer, R. Heart development in Drosophila and its relationship to vertebrates. Trends Cardiovasc Med. 5, 21-28 (1995).
- Brumby, A. M., Richardson, H. E. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 5, 626-639 (2005).
- Chan, H. Y., Bonini, N. M. Drosophila models of human neurodegenerative disease. Cell Death Differ. 7, 1075-1080 (2000).
- Levine, M., et al. Human DNA sequences homologous to a protein coding region conserved between homeotic genes of Drosophila. Cell. 38, 667-673 (1984).
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 6, 9-23 (2005).
- Bier, E., Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene. 342, 1-11 (2004).
- Noyes, B. E., et al. Identification and expression of the Drosophila adipokinetic hormone gene. Mol Cell Endocrinol. 109, 133-141 (1995).
- Rajan, A., Perrimon, N. Of flies and men: insights on organismal metabolism from fruit flies. BMC Biol. 11, 38(2013).
- Rulifson, E. J., et al. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 296, 1118-1120 (2002).
- Birse, R. T., et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 12, 533-544 (2010).
- Musselman, L. P., et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Models Mech. 4, 842-849 (2011).
- Diop, S. B., Bodmer, R. Gaining Insights into Diabetic Cardiomyopathy from Drosophila. Trends Endocrinol Metab. 26, 618-627 (2015).
- Williams, M. J., et al. The Obesity-Linked Gene Nudt3 Drosophila Homolog Aps Is Associated With Insulin Signaling. Mol Endocrinol. 29, 1303-1319 (2015).
- Morris, S. N., et al. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim Biophys Acta. 1822, 1230-1237 (2012).
- Bodmer, R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 118, 719-729 (1993).
- Erkkila, A., et al. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res. 47, 172-187 (2008).
- Ganz, M., et al. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 20, 8525-8534 (2014).
- Wang, C. Y., Liao, J. K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 821, 421-433 (2012).
- Stocker, H., Gallant, P. Getting started: an overview on raising and handling Drosophila. Methods Mol Biol. 420, 27-44 (2008).
- Mathews, K. W., et al. Sexual Dimorphism of Body Size Is Controlled by Dosage of the X-Chromosomal Gene Myc and by the Sex-Determining Gene tra in Drosophila. Genetics. 205, 1215-1228 (2017).
- Golay, A., Bobbioni, E. The role of dietary fat in obesity. Int J Obes Relat Metab Disord. 21, Suppl 3 2-11 (1997).
- Diop, S. B., et al. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 10, 1-13 (2015).
- Chatterjee, D., et al. Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes. Proc Natl Acad Sci U S A. 111, 17959-17964 (2014).
- Palanker, L., et al. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 9, 228-239 (2009).
- Heinrichsen, E. T., Haddad, G. G. Role of high-fat diet in stress response of Drosophila. PLoS One. 7, 42587(2012).
- Kitahara, C. M., et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 11, 1001673(2014).
- Reis, A., et al. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J Lipid Res. 54, 1812-1824 (2013).
- Turne, C., et al. Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J Chromatogr A. 936, 215-237 (2001).
- Na, J., et al. Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 9, 1003175(2013).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved