A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Near-Infrared Temperature Measurement Technique for Water Surrounding an Induction-heated Small Magnetic Sphere
In This Article
Summary
A technique utilizing wavelengths of 1150 and 1412 nm to measure the temperature of water surrounding an induction-heated small magnetic sphere is presented.
Abstract
A technique to measure the temperature of water and non-turbid aqueous media surrounding an induction-heated small magnetic sphere is presented. This technique utilizes wavelengths of 1150 and 1412 nm, at which the absorption coefficient of water is dependent on temperature. Water or a non-turbid aqueous gel containing a 2.0-mm- or 0.5-mm-diameter magnetic sphere is irradiated with 1150 nm or 1412 nm incident light, as selected using a narrow bandpass filter; additionally, two-dimensional absorbance images, which are the transverse projections of the absorption coefficient, are acquired via a near-infrared camera. When the three-dimensional distributions of temperature can be assumed to be spherically symmetric, they are estimated by applying inverse Abel transforms to the absorbance profiles. The temperatures were observed to consistently change according to time and the induction heating power.
Introduction
A technique to measure temperature near a small heat source within a medium is required in many scientific research fields and applications. For example, in the research on magnetic hyperthermia, which is a cancer therapy method using electromagnetic induction of magnetic particles, or small magnetic pieces, it is critical to accurately predict the temperature distributions generated by the magnetic particles1,2. However, although microwave3,4, ultrasound5,6,7,8, optoacoustic9, Raman10, and magnetic resonance11,12-based temperature measurement techniques have been researched and developed, such an inner temperature distribution cannot be accurately measured at present. Thus far, single-position temperatures or temperatures at a few positions have been measured via temperature sensors, which, in the case of induction heating, are non-magnetic optical fiber temperature sensors13,14. Alternatively, the surface temperatures of media have been remotely measured via infrared radiation thermometers to estimate the inner temperatures14. However, when a medium containing a small heat source is a water layer or a non-turbid aqueous medium, we have demonstrated that a near-infrared (NIR) absorption technique is useful to measure the temperatures15,16,17,18,19. This paper presents the detailed protocol of this technique and representative results.
The NIR absorption technique is based on the principle of temperature dependence of the absorption bands of water in the NIR region. As is shown in Figure 1a, the ν1 + ν2 + ν3 absorption band of water is observed in the 1100-nm to 1250-nm wavelength (λ) range and shifts to shorter wavelengths as the temperature increases19. Here, ν1 + ν2 + ν3 means that this band corresponds to the combination of the three fundamental O-H vibration modes: symmetric stretching (ν1), bending (ν2), and antisymmetric stretching (ν3)20,21. This change in the spectrum indicates that the most temperature-sensitive wavelength in the band is λ ≈ 1150 nm. Other absorption bands of water also exhibit similar behavior with respect to the temperature15,16,17,18,20,21. The ν1 + ν3 band of water observed within the range λ = 1350−1500 nm and its temperature dependence are shown in Figure 1b. In the ν1 + ν3 band of water, 1412 nm is the most temperature-sensitive wavelength. Thus, it is possible to obtain two-dimensional (2D) temperature images by using an NIR camera to capture 2D absorbance images at λ = 1150 or 1412 nm. As the absorption coefficient of water at λ = 1150 nm is smaller than that at λ = 1412 nm, the former wavelength is suitable for approximately 10-mm-thick aqueous media, while the latter is suitable for approximately 1-mm-thick ones. Recently, using λ = 1150 nm, we obtained the temperature distributions in a 10-mm-thick water layer containing an induction-heated 1-mm-diameter steel sphere19. Moreover, the temperature distributions in a 0.5-mm-thick water layer have been measured by using λ = 1412 nm15,17.
An advantage to the NIR-based temperature imaging technique is that it is simple to setup and implement because it is a transmission-absorption measurement technique and needs no fluorophore, phosphor, or other thermal probe. In addition, its temperature resolution is less than 0.2 K15,17,19. Such a good temperature resolution cannot be achieved by other transmission techniques based on interferometry, which have often been used in heat and mass transfer studies22,23,24. We note, however, that the NIR-based temperature imaging technique is not suitable in cases with considerable local temperature change, because the deflection of light caused by the large temperature gradient becomes dominant19. This matter is referred in this paper in terms of practical use.
This paper describes the experimental setup and procedure for the NIR-based temperature imaging technique for a small magnetic sphere heated via induction; additionally, it presents the results of two representative 2D absorbance images. One image is of a 2.0-mm-diameter steel sphere in a 10.0-mm-thick water layer that is captured at λ = 1150 nm. The second image is of a 0.5-mm-diameter steel sphere in a 2.0-mm-thick maltose syrup layer that is captured at λ = 1412 nm. This paper also presents the calculation method and results of the three-dimensional (3D) radial distribution of temperature by applying the inverse Abel transform (IAT) to the 2D absorbance images. The IAT is valid when a 3D temperature distribution is assumed to be spherically symmetric as in the case of a heated sphere (Figure 2)19. For the IAT calculation, a multi-Gaussian function fitting method is employed here, because the IATs of Gaussian functions can be obtained analytically25,26,27,28,29 and fit well to monotonically decreasing data; this includes experiments employing thermal conduction from a single heat source.
Protocol
1. Experimental Setup and Procedures
Prepare an optical rail to mount a sample and optics for NIR imaging as follows.
- Sample preparation.
Note: When using water or aqueous liquid, do Step 1.1.1. When using an aqueous gel with high viscosity, do Step 1.1.2.- Steel sphere setting in water.
- Fix a 2.0-mm-diameter steel sphere to the end of a thin plastic string using a small amount of glue.
- Hang the steel sphere at the center of the rectangular glass cell with an optical path length of 10.0 mm, a width of 10 mm, and a height of 45 mm (Figure 3).
- Pour filtered water into the cell carefully so as not to produce air bubbles.
NOTE: A steel sphere can also be fixed to the tip of a thin plastic rod with a small amount of glue19.
- Steel sphere setting in aqueous gel.
- Heat an aqueous gel to reduce its viscosity such that it is low enough to be poured smoothly.
- Using a syringe, pour the aqueous gel into a rectangular glass cell with an optical path length of 2.0 mm, a width of 10 mm, and a height of 45 mm to half-full and leave it to cool.
- Place a 0.5-mm-diameter steel sphere in the center of the gel surface.
- Fill the cell with the aqueous gel.
NOTE: Larger spheres (>~1 mm dia.) should not be used with a gel because they will move by gravitational and/or magnetic forces during induction heating.
- Set the cell in a plastic holder and mount it on the optical rail (Figure 3).
- Steel sphere setting in water.
- Preparation of NIR imaging system.
- Prepare a halogen lamp with a fiber light guide, and fix the end of the fiber light guide with a holder on the optical rail.
- Place a narrow bandpass filter (NBPF) with a transmittance peak at λ = 1150 nm or λ = 1412 nm between the fiber light guide and the cell (Figure 3).
- Interpose another bandpass filter (BPF), whose transmission wavelength range is wider than that of the NBPF, between the halogen lamp and the NBPF.
NOTE: The BPF is needed to prevent thermal damage to the NBPF because it receives light directly. - Interpose an iris diaphragm(s) in the light path between the NBPF and cell holder to reduce the stray light (Figure 3).
- Set up an NIR camera to detect the light transmitted through the cell (Figure 3). Connect the camera through a data transfer cable to a graphic board installed in a personal computer (PC) with image acquisition software.
- Set a telecentric lens between the cell and camera (Figure 3).
NOTE: A common camera lens can also be used. However, a telecentric lens is better in terms of the selective detection of the light parallel to the chief ray for the IAT and reduction of the influence of diffraction.
NOTE: The NBPF and BPF should not be placed between the cell and camera because, in doing so, the water temperature would increase via direct absorption of high-intensity light from the halogen lamp. - Turn on the NIR camera and launch the image acquisition software.
- Light the halogen lamp and adjust its output power observing the image displayed on the monitor (Figure 4).
- Adjust the axis, position, and focus of the telecentric lens to obtain a fine image of the steel sphere.
NOTE: If the adjustment is not complete, irregular intensity patterns will appear, leading to incorrect absorbances.
- Preparation of induction heating system.
- Prepare an induction heating system consisting of a high-frequency generator (maximum output power: 5.6 kW; frequency: 780 kHz), water-cooled coil, and water chiller.
NOTE: An induction heating system for brazing, welding, and soldering small metal parts is appropriate for this purpose; see Table of Materials. - If possible, mount the coil on an XYZ movable stage to change its position.
- Place the coil near the cell such that the distance between the coil center and the steel sphere is approximately 15 mm (Figure 3). Ensure that there are no other metal parts near the coil.
NOTE: The distance should be adjusted depending on the induction heating power and the sphere size. - Circulate water for cooling.
- Prepare an induction heating system consisting of a high-frequency generator (maximum output power: 5.6 kW; frequency: 780 kHz), water-cooled coil, and water chiller.
- Image acquisition and induction heating.
- Click "start" on the image acquisition software to store the images sequentially.
- Click "start" on the induction heating control software to commence the induction heating.
- After several seconds (depending on the conditions and purpose), click "stop" on the image acquisition software.
- Click "stop" on the induction heating control software.
- Save the temporally-stored images as a TIFF sequence (or other non-compressed format) on the image acquisition software.
NOTE: If the temperature is high enough, the effect of light deflection will appear on the image7. The induction heating power must be decreased appropriately though experiments such that the increase in the temperature near the sphere is less than approximately 10 K, which can be confirmed in the following protocol steps for temperature estimation.
2. Image Processing and Temperature Estimation
Note: The saved sequential images are represented as Ii(x, z), where i is the sequential frame number. The coordinates, x, y, z, r, and r' are defined as are indicated in Figure 2; z is positive in the direction opposite to gravity. The outline of the following protocol steps is also illustrated in Supplement 1.
- Absorbance image construction.
- Open Ii(x, z) with the image processing software.
- Reduce noise in Ii(x, z) by implementing 3 × 3 pixel averaging.
- Create an average image of Ii(x, z) over i = 1 to 5 (or more) before heating, and define it as the reference image, Ir(x, z).
NOTE: This averaging reduces the noise to obtain a more reliable image than a single frame image. - Construct the sequential images of the absorbance difference, ΔAi(x, z), via the following equation:
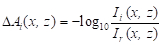 (1)
(1)
NOTE: ΔAi(x, z) is the variation in the absorbance, Ai(x, z), from the reference absorbance, Ar(x, z), before heating, and is derived as follows15,16,17,18,19:
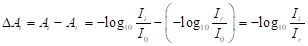 (2)
(2)
where I0 is the intensity of incident light to the cell. - Colorize the ΔAi images using an appropriate color map such as blue-to-red.
NOTE: The command script file for running Steps 2.1.2 through 2.1.5 for ImageJ is presented in Supplement 2.
- Temperature estimation.
- Choose the time period during which ΔAi(x, z) is circularly symmetric with respect to the center of the sphere by visually observing the images.
NOTE: The circular symmetry is broken mainly by free convection. An image-based analytical judgement of free convection occurring is introduced in the previous work19; however, practically, the visual judgement is effective. - Extract the ΔAi(rʹ, θ) data along 360 radial lines (Δθ = 1˚) on the ΔAi(x, z) images.
- Exclude the ΔAi(rʹ, θ) data within the sphere and in its vicinity (Δrʹ≈ 0.2 mm). Note: The data are anomalously very small or large in the vicinity mainly because of the slight movement of the sphere.
- Average ΔAi(rʹ, θ) over θ to determine the line profile, ΔAi(rʹ).
NOTE: The command script file for running Steps 2.2.2 through 2.2.4 for ImageJ is presented in Supplement 3. - Approximate the ΔAi(rʹ) data by the following multi-Gaussian function:
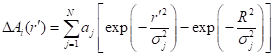 (3)
(3)
where aj is the weighting factor, σj is the dispersion parameter, and R is the maximum of rʹ where ΔAi(R) = 0 can be assumed. - Calculate the absorption coefficient difference, Δµi(r), by substituting the obtained N, aj, and σj into the following IAT of Eq. (3):
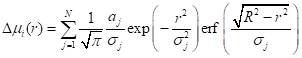 (4)
(4)
where erf is the error function. - Convert Δµi(r) to temperature via the following equation:
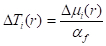 (5)
(5)
with the temperature coefficients of water, αf, which are 2.8 x 10-4 K-1 mm-1 for λ = 1150 nm19 and 4.1 × 10-3 K-1 mm-1 for λ = 1412 nm17.
Note: The command script file for running Steps 2.2.5 through 2.2.7 is presented in Supplement 4, where the Levenberg-Marquardt nonlinear least-squares algorithm17,19 is employed for Step 2.2.5.
- Choose the time period during which ΔAi(x, z) is circularly symmetric with respect to the center of the sphere by visually observing the images.
Results
Images of ΔAi(x, z) at λ = 1150 nm for a 2.0-mm-diameter steel sphere in water and at λ = 1412 nm for a 0.5-mm-diameter steel sphere in maltose syrup are presented in Figure 5a and Figure 6a, respectively. In both cases, the sphere was located 12 mm below the bottom of the coil along its central axis. Figu...
Discussion
The technique presented in this paper is a novel one using the temperature dependence of NIR absorption of water and presents no significant difficulty in setting up the necessary equipment and implementation. The incident light can be easily produced by using a halogen lamp and an NBPF. However, lasers cannot be used, because coherent interference patterns would appear on the images. Common optical lenses and glass cells for visible-light use can be used, as they transmit an adequate amount of light at λ =...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Mr. Kenta Yamada, Mr. Ryota Fujioka, and Mr. Mizuki Kyoda for their support on the experiments and data analyses. This work was supported by JSPS KAKENHI Grant Number 25630069, the Suzuki Foundation, and the Precise Measurement Technology Promotion Foundation, Japan.
Materials
| Name | Company | Catalog Number | Comments |
| Induction heating system | CEIA, Italy | SPW900/56 | 780 kHz, 5.6 kW (max). |
| Coil | SA-Japan | custom | Water-cooled copper tube; two-turn; outer dia. 28 mm. |
| Water chiller | Matsumoto Kikai, Japan | MP-401CT | |
| Halogen lamp | Hayashi Watch-Works, Japan | LA-150UE-A | |
| Narrow bandpass filter for λ = 1150 nm | Andover | 115FS10-25 | Full width at half-maximum (FWHM): 10 nm. |
| Narrow bandpass filter for λ = 1412 nm | Andover | semi-custom | Full width at half-maximum (FWHM): 10 nm. |
| Bandpass filter for λ = 850−1300 nm | Spectrogon | SP-1300 | |
| Bandpass filter for λ = 1100−2000 nm | Spectrogon | SP-2000 | |
| NIR camera | FLIR Systems | Alpha NIR | InGaAs |
| Image acquisition software | FLIR Systems | IRvista | |
| Image processing software | NIH | ImageJ | ver. 1.51r |
| Image processing software | MathWorks | Matlab | ver. 2016a |
| Telecentric lens | Edmond Optics | 55350-L | X1 |
| Steel sphere (0.5 mm dia.) | Kobe Steel, Japan | Fe-1.5Cr-1.0C-0.4Mn (wt %) | |
| Steel sphere (2.0 mm dia.) | Kobe Steel, Japan | Fe-1.5Cr-1.0C-0.4Mn (wt %) | |
| Maltose syrup as aqueous gel | Sonton, Japan | Mizuame | Food product |
References
- Moros, E. G. . Physics of Thermal Therapy. , (2012).
- Périgo, E. G., Hemery, G., Sandre, O., Ortega, D., Garaio, E., Plazaola, F., Teran, F. J. Fundamentals and advances in magnetic hyperthermia. Appl Phys Rev. 2, 041302 (2015).
- Bardati, F., Marrocco, G., Tognolatti, P. Time-dependent microwave radiometry for the measurement of temperature in medical applications. IEEE Trans Microwave Theo Tech. 52, 1917-1924 (2004).
- Levick, A., Land, D., Hand, J. Validation of microwave radiometry for measuring the internal temperature profile of human tissue. Meas Sci Technol. 22, 065801 (2011).
- Daniels, M. J., Varghese, T., Madsen, E. L., Zagzebski, J. A. Non-invasive ultrasound-based temperature imaging for monitoring radiofrequency heating-phantom results. Phys Med Biol. 52, 4827 (2007).
- Daniels, M. J., Varghese, T. Dynamic frame selection for in vivo ultrasound temperature estimation during radiofrequency ablation. Phys Med Biol. 55, 4735 (2010).
- Seo, C. H., Shi, Y., Huang, S. -. W., Kim, K., O'Donnell, M. Thermal strain imaging: A review. Interface Focus. 1, 649-664 (2011).
- Bayat, M., Ballard, J. R., Ebbini, E. S. Ultrasound thermography: A new temperature reconstruction model and in vivo results. AIP Conf Proc. 1821, 060004 (2017).
- Petrova, E., Liopo, A., Nadvoretskiy, V., Ermilov, S. Imaging technique for real-time temperature monitoring during cryotherapy of lesions. J Biomed Opt. 21, 116007 (2016).
- Gardner, B., Matousek, P., Stone, N. Temperature spatially offset Raman spectroscopy (T-SORS): Subsurface chemically specific measurement of temperature in turbid media using anti-Stokes spatially offset Raman spectroscopy. Anal Chem. 88, 832-837 (2016).
- Yoshioka, Y., Oikawa, H., Ehara, S., Inoue, T., Ogawa, A., Kanbara, Y., Kubokawa, M. Noninvasive measurement of temperature and fractional dissociation of imidazole in human lower leg muscles using 1H-nuclear magnetic resonance spectroscopy. J Appl Physiol. 98, 282-287 (2004).
- Galiana, G., Branca, R. T., Jenista, E. R., Warren, W. S. Accurate temperature imaging based on intermolecular coherences in magnetic resonance. Science. 322, 421-424 (2008).
- Rapoport, E., Pleshivtseva, Y. . Optimal Control of Induction Heating Processes. , (2006).
- Lucía, O., Maussion, P., Dede, E. J., Burdío, J. M. Induction heating technology and its applications: Past developments, current technology, and future challenges. IEEE Trans Ind Electron. 61, 2509-2520 (2014).
- Kakuta, N., Kondo, K., Ozaki, A., Arimoto, H., Yamada, Y. Temperature imaging of sub-millimeter-thick water using a near-infrared camera. Int J Heat Mass Trans. 52, 4221-4228 (2009).
- Kakuta, N., Fukuhara, Y., Kondo, K., Arimoto, H., Yamada, Y. Temperature imaging of water in a microchannel using thermal sensitivity of near-infrared absorption. Lab Chip. 11, 3479-3486 (2011).
- Kakuta, N., Kondo, K., Arimoto, H., Yamada, Y. Reconstruction of cross-sectional temperature distributions of water around a thin heating wire by inverse Abel transform of near-infrared absorption images. Int J Heat Mass Trans. 77, 852-859 (2014).
- Kakuta, N., Yamashita, H., Kawashima, D., Kondo, K., Arimoto, H., Yamada, Y. Simultaneous imaging of temperature and concentration of ethanol-water mixtures in microchannel using near-infrared dual-wavelength absorption technique. Meas Sci Technol. 27, 115401 (2016).
- Kakuta, N., Nishijima, K., Kondo, K., Yamada, Y. Near-infrared measurement of water temperature near a 1-mm-diameter magnetic sphere and its heat generation rate under induction heating. J Appl Phys. 122, 044901 (2017).
- Libnau, F. O., Kvalheim, O. M., Christy, A. A., Toft, J. Spectra of water in the near- and mid-infrared region. Vib Spectrosc. 7, 243-254 (1994).
- Siesler, H. W., Ozaki, Y., Kawata, S., Heise, H. M. . Near-Infared Spectroscopy. , (2002).
- Shakher, C., Nirala, A. K. A review on refractive index and temperature profile measurements using laser-based interferometric techniques. Opt Laser Eng. 31, 455-491 (1999).
- Assebana, A., Lallemanda, M., Saulniera, J. -. B., Fominb, N., Lavinskaja, E., Merzkirchc, W., Vitkinc, D. Digital speckle photography and speckle tomography in heat transfer studies. Opt Laser Technol. 32, 583-592 (2000).
- Ambrosini, D., Paoletti, D., Spagnolo, S. G. Study of free-convective onset on a horizontal wire using speckle pattern interferometry. Int J Heat Mass Trans. 46, 4145-4155 (2003).
- Bracewell, R. N. . The Fourier Transform and Its Applications. , (2000).
- Yoder, L. M., Barker, J. R., Lorenz, K. T., Chandler, D. W. Ion imaging the recoil energy distribution following vibrational predissociation of triplet state pyrazine-Ar van der Waals clusters. Chem Phys Lett. 302, 602-608 (1999).
- De Colle, F., de Burgo, C., Raga, A. C. Diagnostics of inhomogeneous stellar jets: convolution effects and data reconstruction. Astron Astrophys. 485, 765-772 (2008).
- Green, K. M., Borrás, M. C., Woskov, P. P., Flores, G. J., Hadidi, K., Thomas, P. Electronic excitation temperature profiles in an air microwave plasma torch. IEEE Trans Plasma Sci. 29, 399-406 (2001).
- Bendinelli, O. Abel integral equation inversion and deconvolution by multi-Gaussian approximation. Astrophys J. 366, 599-604 (1991).
- Dorband, B., Muller, H., Gross, H., Gross, H. Vol. 5 Metrology of Optical Components and Systems. Handbook of Optical System. , (2012).
- Sheikholeslami, M., Rokni, H. B. Simulation of nanofluid heat transfer in presence of magnetic field: A review. Int J Heat Mass Trans. 115, 1203-1233 (2017).
- Häfeli, U., Schütt, W., Teller, J., Zborowski, M. . Scientific and Clinical Applications of Magnetic Carriers. , (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved