Method Article
A Visual Guide for Studying Behavioral Defenses to Pathogen Attacks in Leaf-Cutting Ants
In This Article
Summary
We present a visual guide to disease defense behaviors in leaf-cutting ants, with individual clips and accompanying definitions, illustrated in an experimental infection scenario. Our main aim is to help other researchers recognize key defensive behaviors and to provide a common understanding for future research in this field.
Abstract
The complex lifestyle, evolutionary history of advanced cooperation, and disease defenses of leaf-cutting ants are well studied. Although numerous studies have described the behaviors connected with disease defense, and the associated use of chemicals and antimicrobials, no common visual reference has been made. The main aim of this study was to record short clips of behaviors involved in disease defense, both prophylactically and directly targeted towards an antagonist of the colony following infection. To do so we used an infection experiment, with sub-colonies of the leaf-cutting ant species Acromyrmex echinatior, and the most significant known pathogenic threat to the ants' fungal crop (Leucoagaricus gongylophorus), a specialized pathogenic fungus in the genus Escovopsis. We filmed and compared infected and uninfected colonies, at both early and more advanced stages of infection. We quantified key defensive behaviors across treatments and show that the behavioral response to pathogen attack likely varies between different castes of worker ants, and between early and late detection of a threat. Based on these recordings we have made a library of behavioral clips, accompanied by definitions of the main individual defensive behaviors. We anticipate that such a guide can provide a common frame of reference for other researchers working in this field, to recognize and study these behaviors, and also provide greater scope for comparing different studies to ultimately help better understand the role these behaviors play in disease defense.
Introduction
Leaf-cutting ants are advanced social insects, forming some of the most complex colonies on earth. They are a derived branch of the fungus-growing ants (tribe Attini) consisting of the two genera Acromyrmex and Atta1. They cultivate the fungal crop species Leucoagaricus gongylophorus (Basidiomycota: Agaricales), which they rely on as their main food source2,3. The ants supply this fungus with fresh leaf material for its growth, and the fungus in return produces nutrient-rich swollen hyphal tips (gongylidia) that are consumed by the ants and their brood. Colonies are built underground, the fungal crop is maintained in external gardens4,5, and the ant farmers protect their crop monoculture from potential pathogens6,7,8,9,10,11,12. Colonies divide labor between workers of different size (caste) and age13,14,15, which extends to the defense of ants and crop from pathogens.
We might expect that leaf-cutting ant colonies should be vulnerable to disease. Group living is expected to facilitate spread of diseases between related workers due to frequent interactions and, thus, easier transmission16. The ants are susceptible to entomopathogenic fungal parasites, such as Metarhizium species and Beauveria bassiana6. These parasites are generalists and are often present in the soil close to the nests7,8. Farming of the fungus crop as a monoculture4,5 makes it likely to also be susceptible to disease17,18. It can be infected by generalist fungal parasites (including Aspergillus niger and Trichoderma species3); however, the most significant threat is a specialist necrotrophic fungus in the genus Escovopsis (Ascomycota: Hypocreales)11. Through secretion of mycolytic enzymes and other compounds, Escovopsis kills and obtains nutrients from the fungus crop12, with potentially fatal consequences for the ant colonies11,19.
To combat disease threats, the ants have remarkable defenses at both individual and colony level, combining biological control, behavioral and chemical defenses to act prophylactically and, when necessary, in response to infection. Collectively, these defenses prevent or reduce the impact of infections from both generalist pathogens and specialists such as Escovopsis. Broadly they involve avoiding the contraction of parasites in the environment20, preventing parasites from entering the nest, and limiting the spread of infection within nests. The first lines of defense include chemicals from glandular secretions3,21,22,23,24,25,26,27 to disinfect plant substrates, through workers licking them prior to incorporation in the fungus garden, and ants carrying out both self- and allogrooming. When grooming themselves, especially upon entering the nest, workers may also apply acidic fecal secretions to their body27. These prophylactic defenses are demonstrably important to avoid infection by pathogenic threats6,7,8,9,10,11,12.
If initial defenses fail and a pathogen such as Escovopsis succeeds in entering the nest and the fungus garden, and if infection is detected at an early stage, the ants use fungus grooming to remove spores25,28. The ants may apply secretions from the metapleural glands or transfer the spores to the infrabuccal pocket (an oral cavity), where they are mixed with a chemical cocktail containing metapleural and labial gland secretions26. There are more than 20 known compounds in these glands, including γ-keto-, carboxylic- and indoleactic acids3. These are actively applied25, have antibiotic, fungistatic and fungicidal properties29, and can inhibit Escovopsis spore germination30. Spores stored in the infrabuccal pocket are later expelled outside the colony31,32. Most of this fungus grooming following early-stage detection is carried out by minor workers28,33. However, if a pathogen manages to avoid detection and spreads within the fungus garden, both minor and major workers weed infected parts of the fungus28, and this removed material is deposited outside the nest31. In addition, species in the leaf-cutting genus Acromyrmex use biological control in the form of antibiotics produced by symbiotic Actinobacteria34,35,36— maintained on the ant cuticle37of predominantly young major workers34,38,39,40— to produce compounds that prevent mycelial growth of Escovopsis34,38,41. This antibiotic production may in turn be impaired by Escovopsis-produced compounds during an infection19.
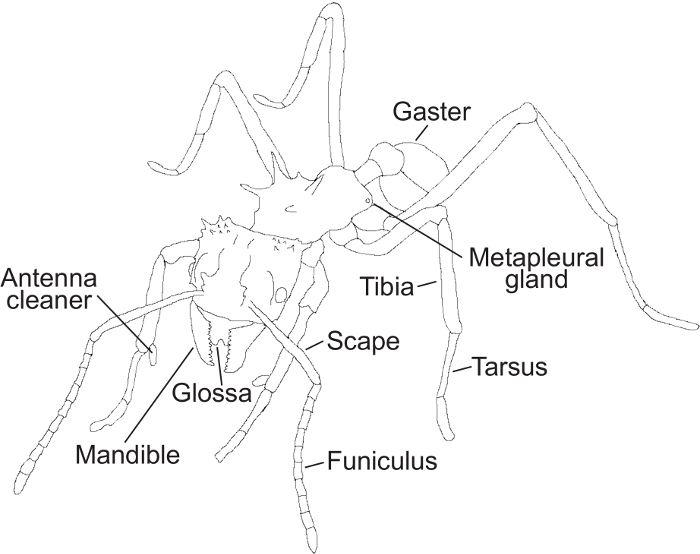
Figure 1: Ant morphological features. A schematic drawing of an ant showing the morphological structures mentioned in the protocol. Please click here to view a larger version of this figure.
Leaf-cutting ant defenses thus constitute an integrated assembly of behavioral and chemical mechanisms that collectively provide these ants with very efficient protection from disease42. Understanding these defenses is of broad interest, and they have been extensively researched16,20,42,43,44. However, a visual compilation of the defensive behaviors that would help unambiguous definition and description of them for systematic use by researchers is, to our knowledge, not available. Although the terminology used to describe ant behavior is relatively standardized, there is therefore no certainty that the same behavioral acts are named consistently in different studies. Here, our main goal is to remedy this by providing clarity and standardization through a compilation of video recordings of individual prophylactic and defensive behaviors accompanied by associated definitions. We filmed these clips during a behavioral experiment, in which we observed and quantified behaviors in the context of experimental Escovopsis infections of Acromyrmex echinatior sub-colonies, the results of which we also present here as an illustrative example of how this compilation can be used for behavioral studies.
Protocol
1. Escovopsis isolation
- Isolate Escovopsis strains from Acromyrmex echinatior laboratory colonies, by placing fungus garden fragments, with ants removed, on a Petri dish with moist cotton wool for several days until Escovopsis germinates and sporulates. Transfer spores to potato dextrose agar plates (PDA, 39 g/L) and incubate at ca. 23°C for approximately two weeks.
Note: One strain was used in the current study. - Use a sterile needle to pick out mature spores from these first plates. Pick enough spores to cover the tip of the needle.
- Inoculate spores on new plates under sterile conditions and incubate at ~ 23 °C for approximately two weeks.
- When hyphae have covered the entire plate and grown into mature brown spores, repeat step 1.3 but this time with spores from the new PDA plate.
- Repeat the process until a clean stock of Escovopsis for each strain is acquired (no visible contaminants growing on or below the plate surface).
2. Experimental set-up
- Use three A. echinatior colonies.
Note: Here the colonies Ae160b, Ae322 and Ae263 were used. - From each colony, make 12 sub-colonies. Make six sub-colonies for observation after 0 h and six sub-colonies for observation after 72 h. This gives a total of 36 sub-colonies; mark half of the sub-colonies from each ‘parent’ colony as control, and the other half as ‘Escovopsis treatment’.
Note: While in parent colonies the presence of a queen affects worker behavior, we expect (although this cannot be guaranteed), that queen-less sub-colonies are likely to behave as queenright colonies for the short period of time that the experiment runs. - For each sub-colony, use a square box of length: ~ 3.15 in (8 cm), width: ~ 2.17 in (5.5 cm) and depth ~ 1.77 in (4.5 cm).
Note: The important point is to provide enough space for ants outside the fungus fragment to forage and dump waste, but at the same time to make the box small enough that filming and behavior recognition is feasible. - For each sub-colony, add a tea-spoon sized (approximately 2.2 cm3 and 1.2 g) piece of the central part of the fungus garden (L. gongylophorus) from the original colony, a couple of bramble leaves, and a piece of cotton wool soaked in water.
Note: The cotton wool provides humidity. It should not be dripping with water, and should not touch the fungus garden. - For sub-colonies treated with Escovopsis, use an inoculation loop and fill the opening so it is just covered by Escovopsis spores. Inoculate the spores by gently tapping a small, localized part of the fungus garden ten to twenty times so that spores are not too clustered.
- For sub-colonies used as controls, mimic the application of Escovopsis to the fungus garden with a sterile inoculation loop.
Note: Although this was not done in the present experiment, the inoculation of a sterile powder (like graphite or talcum powder) can be done at this stage to differentiate between an infection with a pathogen and an inert agent. - For 72 h observations, leave half of the sub-colonies (both controls and infected) for 72 h after introduction of Escovopsis before adding ants or initiating video recording.
Note: Waiting 72 h in the absence of ants makes it likely that Escovopsis spores will germinate (unpublished in vitro data); although this also increases the chance of other infections (for example, from a fungus already present in the fungus garden), this time period is preferable for this treatment to represent the early stage of an established infection. - For 0 h observations, directly after step 2.6 and approximately 30 min before recording, add two brood, four minor workers and four major workers simultaneously from the parent colony to each box.
- Use two major workers from within the garden that are young with light pigment, with Actinobacteria covering most of the cuticle. Take the other two from outside the garden, with dark pigment and Actinobacteria only covering the laterocervical plates.
- For 72 h observations, repeat 2.8 and 2.8.1 at 30 min before recording, i.e., 71.5 h after inoculation with Escovopsis.
Note: Experimental sub-colonies are significantly smaller than established natural Acromyrmex colonies. This is necessary in order to accurately record behavior. While this may influence the frequency of some behaviors qualitatively, the composition of sub-colonies was chosen to reflect the mix of workers in natural colonies to more likely qualitatively reflect the behavioral interactions.
3. Video recording and scoring behaviors
- Use a USB endoscope attached to a laptop (or equivalent) and provide sufficient light.
- For each sub-colony, perform video recording for 4 h (starting at either 0 h or 72 h post-infection).
- After recording the 36 sub-colonies, review the total of 144 h of footage and score all behaviors of interest for all individuals in each sub-colony.
Note: In the current experimental example, we had to exclude two sub-colonies (a control from colony Ae160b and a treatment sub-colony from colony Ae322) due to infection with fungi other than Escovopsis, reducing the total number of hours of observations to 136. - Each time a behavior is observed, record it as 1 occurrence.
Note: A behavior can be of short or long duration, but only score it as > 1 if it is interrupted by another behavior, or if the ant is passive for a period of time.
4. Behaviors
Note: Behavioral definitions were made using a combination of descriptions from previous studies23,27,28,31,45 and personal observations. For a detailed illustration showcasing important morphological structures used in the protocol for recognition of behaviors, see Figure 1.
- Self-grooming and antenna cleaning (Video 1)
- Notice if the ant stops leg movement to initiate self-grooming. Check that the antennae are pulled through the antenna cleaners on the front legs (Figure 1), a clamp-like structure located on the tibia-tarsus joint consisting of a notch facing a spur with different sized bristles and combs45,46.
- After cleaning the antenna, observe that the ant will clean the legs and the antenna cleaners, by pulling the legs through the mouthparts, removing particles and potential pathogens with the glossa (Figure 1).
Note: Self-grooming can consist of cleaning the antenna and subsequently the antenna cleaners (Figure 1), but also using mouthparts to clean the legs. When an ant is cleaning its legs, it most often cleans all six legs in succession.
- Fungus grooming (Video 2)
- Notice if the ant stops leg movements at a fixed point on the fungus garden. Observe that the antennae are motionless and parallel pointed towards the fungus so that the angle between the scape and funiculus (Figure 1) is approximately 45°, and the tip of the antennae are almost touching each other, close to the tips of the mandibles (Figure 1).
- Note that the upper (maxillae) and lower (labium) mouthparts are open, with the glossa (Figure 1) emerging to lick the fungus.
- Allogrooming (Video 3)
- Observe this behavior when one or more ants have approached another (recipient) ant or vice versa. During the behavior, the ants stop movement and stand closely together with physical contact. The grooming ant(s) may move slightly to cover a larger area of the recipient ant’s body.
- Observe that the antennae of the actor(s) can be motionless and pointed towards a specific point of the receiving ant or moving and lightly tapping the receiver. The angle between the scape and the funiculus (Figure 1) is approximately 45° depending on whether they are fixed on a specific point or tapping. The tips of the actor’s antennae are usually close to each other and the tips of the mandibles (Figure 1).
- Note that the upper (maxillae) and lower (labium) mouthparts are open, with the glossa (Figure 1) emerging to lick the receiver ant.
- Metapleural gland grooming (Video 4)
- Observe when the ant stops movement to initiate metapleural gland (Figure 1) grooming. Note that the ant leans to one side to reach one of its front legs back to rub the opening (meatus) of the metapleural gland (for example, the right front leg).
Note: The other front leg is simultaneously (in this example the left front leg) licked by the glossa (Figure 1). - Check that the ant leans to the opposite side and switches legs and repeats the same motion with the opposite legs. The ant continues to move the front legs to the metapleural glands and subsequently to the glossa constantly switching between legs (Figure 1).
Note: In this example, the ant will now pass the left front leg to the metapleural gland and the right front leg to the glossa (Figure 1). After metapleural gland (Figure 1) grooming, the ant often initiates self-grooming (Step 4.1).
- Observe when the ant stops movement to initiate metapleural gland (Figure 1) grooming. Note that the ant leans to one side to reach one of its front legs back to rub the opening (meatus) of the metapleural gland (for example, the right front leg).
- Spore weeding (Video 5)
- Observe this behavior when the ant stops leg movements at a fixed point on the fungus garden. Observe that the antennae are motionless and parallel, pointed towards the fungus so that the angle between the scape and funiculus is approximately 45°, and the tips of the antennae are almost touching each other and the tips of the mandibles (Figure 1).
- Check that the ant opens its mandibles (Figure 1) to grab visible Escovopsis spores and detach them from the fungal crop by pulling them off. The ant carries the cluster of spores out of the nest, while the antennae are gently moving for orientation. The antennae may be cleaned through the antenna cleaners (Figure 1; see Video 1) while holding the cluster of spores. The ant drops the spores off in a waste pile.
Note: Recording of activity around the waste pile was not done in the current experiment but would be a suitable extension of the current protocol.
- Fungus weeding (Video 6)
- Observe this behavior when the ant stops leg movements at a fixed point of the fungus. The antennae are loosely pointed towards the part of the fungus the ant is attempting to remove, while slightly tapping the fungus piece.
- Observe that the ant uses its mandibles to either cut through the fungal crop to detach a specified area, or to grab a part of the fungus piece with its mandibles (Figure 1). The ant will simultaneously rock from side to side on its legs, while it pulls off the fungus piece.
Note: Weeding can be done by multiple workers and by both minors and majors. If so, some ants do the fungus cutting, others carry out the rocking and pulling motion. The detached part of the fungus is carried outside the nest and dropped off in the waste pile. Recording of the waste deposit was not done in the current experiment but would be a suitable extension of the current protocol.
- Fecal fluid grooming (Video 7)
- Observe this behavior when the ant stops leg movements at a fixed point on the fungus garden. The ant bends its gaster (Figure 1) and head towards each other to apply a droplet of fecal fluid to the mouthparts.
- Observe that the ant pulls the front legs through the mandibles (Figure 1), one at a time. Subsequently, the ant moves the antennae through the antenna cleaners (Figure 1) located on the tibia-tarsus joint of the front legs.
- Droplet regurgitation (Video 8)
- Observe this behavior when the ant stops leg movements at a fixed point of the fungus. The antennae are motionless and parallel, pointed towards the fungus so that the angle between the scape and funiculus is approximately 45°, and the tips of the antennae almost touch each other and the tips of the mandibles (Figure 1).
- Observe that the ant regurgitates a droplet of liquid on to the fungus, varying from being transparent to light yellow or even brown, from its mouthparts.
Results
The main objective of this study was the creation of short clips that illustrate behaviors associated with disease defense in leaf-cutting ants, to generate a catalogue to be used as a reference for future studies. In addition, the study uses an example of an experimental setup within which these behaviors were quantified to show how this catalogue may be used in behavioral studies, the representative results of which we summarize here.
Sub-colonies were set up for observation at early stage (0 h) and late stage (72 h) infection. Due to heavy infections with fungi other than Escovopsis after 72 h, two sub-colonies (one control for colony Ae160b and one treatment for colony Ae322) were excluded, so we focus the presentation of the results on the 0 h time point and put less emphasis on behaviors observed after 72 h. After filming all sub-colonies and scoring the defensive behaviors, we found differences in behavior patterns associated with time after infection and context, including the different ways in which they were used by minor and major workers. In contrast, self-grooming was performed at all times in both control and infected sub-colonies. It was also common when ants were seeking, and attempting to remove, Escovopsis hyphae or spores. Because this behavior was so universally observed and frequent in all situations, we did not quantify it.
We scored all other behaviors described in the protocol and present calculated total and average frequencies for minor and major workers per sub-colony (Table 1). We found that in controls, minor workers groomed the garden crop more than major workers (Figure 2a), and anecdotally observed that they spent more time in the fungus garden. In colonies infected with Escovopsis there was a tendency for increased fungus grooming overall relative to control colonies, but this was not significant (F1,23 = 2.80, p = 0.1077; Table 2; Figure 2a). There was a non-significant increase in fecal grooming with infection (F1,23 = 0.60, p = 0.4455; Table 1) but no difference between minor and major workers (Figure 2b). We observed fecal grooming when workers entered the fungus, rather than when they were on or completely away from the fungus for extended periods of time.
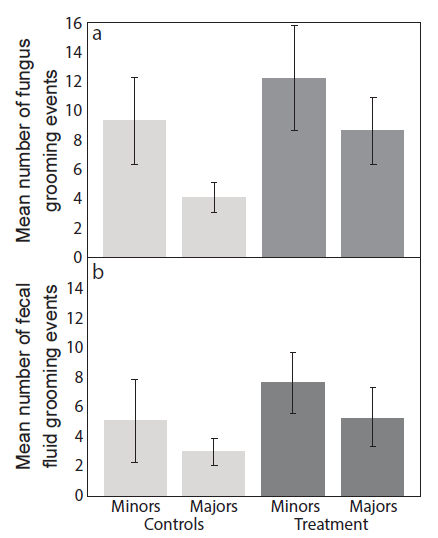
Figure 2: Frequency of grooming events. Mean number (± Standard Errors (SE); n = 9) of (a) fungus grooming and (b) fecal fluid grooming events within four hours after inoculation, comparing minor and major workers in controls and Escovopsis treatments. Please click here to view a larger version of this figure.
Several behaviors were extremely rare. We only observed metapleural gland grooming four times, and all instances occurred after 72 h; once in the control group, and three times in colonies with Escovopsis infection (Table 1). Almost as infrequently, we found that ants would regurgitate a liquid droplet onto the fungus garden and a few times outside of the fungus (Table 1). In the 0 h groups, this happened once in a control sub-colony and twice in infected colonies - in all three instances the behavior was performed by a major worker. On one occasion a minor worker regurgitated a droplet in the corner of the box and once a major worker did the same on a bramble leaf, both in the Escovopsis treatment. In the 72 h colonies, droplet regurgitation on the fungus was never observed in the control colonies but happened seven times in infected colonies. Six of these were by major workers and three of these by a single individual that added droplets outside of the fungus garden.
Spore weeding (Figure 3a) and fungus weeding (Figure 3b) were both low in frequency. While there was no significant difference in the frequency of fungus weeding between infected colonies and controls, there was a tendency for weeding to increase with time since infection (F1,23= 2.91, p = 0.1014; Table 2). Our observations focused on Escovopsis spore weeding, which did not change significantly with time, but there was a tendency for a higher frequency in colonies infected with Escovopsis than in uninfected controls (F1,23= 3.27, P = 0.0838; Table 2). Fewer sub-colonies infected with Escovopsis had visible spores remaining after the observation period with an early-stage infection (when ants were introduced after 0 h of spore inoculation), with ants removing all spores in almost half of the sub-colonies (4 out of 9; Figure 3c). In later stage infections (where ants were introduced after 72 h of spore inoculation), the ants were not capable of removing spores completely in any of the sub-colonies. Taken together, these observations suggest a tendency for ants to shift from spore weeding to fungus weeding over the course of an infection.
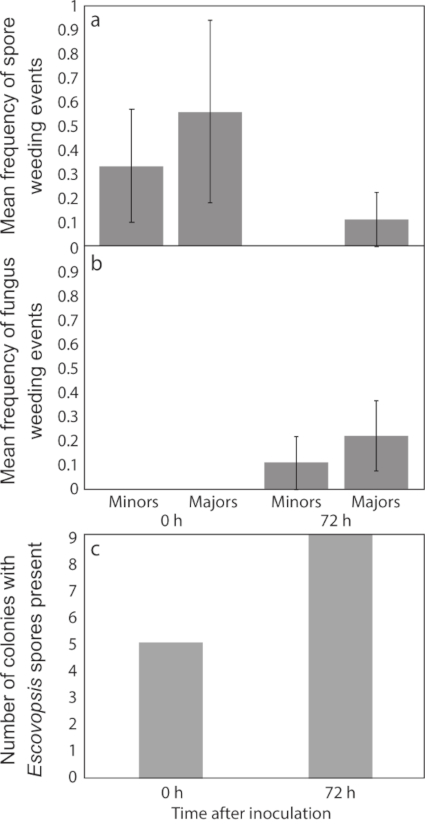
Figure 3: Frequency of weeding events. Mean frequency (± Standard Errors (SE); n = 9) of (a) spore weeding (of Escovopsis spores) and (b) fungus weeding (Escovopsis or other fungi) during a 4 h observation period, comparing minors and majors from treatment groups, and (c) the number of sub-colonies with visible Escovopsis spores in treatment groups at the end of the observation period. Please click here to view a larger version of this figure.
| Minor workers - control | Minor workers - treatment | Major workers - control | Major workers - treatment | |
| 0h | ||||
| Fungus grooming | 84 (9.333) | 131 (14.56) | 37 (4.111) | 66 (7.333) |
| Allogrooming | 6 (0.6667) | 14 (1.556) | 5 (0.5556) | 6 (0.6667) |
| Spore weeding | 0 | 3 | 0 | 5 (0.5556) |
| Fungus weeding | 0 | 0 | 0 | 0 |
| Fecal fluid grooming | 46 (5.111) | 69 (7.667) | 27 (3.000) | 48 (5.333) |
| Droplet application | 0 | 0 | 1 (0.1111) | 2 (0.2220) |
| Metapleural gland grooming | 0 | 0 | 0 | 0 |
| 72h | ||||
| Fungus grooming | 10 (1.429) | 45 (5.000) | 24 (3.429) | 38 (4.222) |
| Allogrooming | 2 (0.2857) | 10 (1.111) | 1 (0.1110) | 4 (0.4440) |
| Spore weeding | 0 | 0 | 0 | 1 (0.1110) |
| Fungus weeding | 0 | 1 (0.1110) | 0 | 2 (0.2220) |
| Fecal fluid grooming | 19 (2.714) | 38 (4.222) | 30 (4.286) | 30 (3.333) |
| Droplet application | 0 | 1 (0.1110) | 0 | 6 (0.6667) |
| Metapleural gland grooming | 0 | 2 (0.2220) | 1 (0.1429) | 1 (0.1110) |
Table 1: The number of behaviors in four hours of observation at 0 and 72 h after Escovopsis inoculation. The mean total number of observations (with mean frequencies per individual in brackets), comparing minor and major workers from control and Escovopsis infection sub-colonies, respectively (n = 9).
| Type 3 tests of fixed effects | |||||||||||||||||||||
| Fungus grooming | Allogrooming | Fecal grooming | Spore weeding | Fungus weeding | |||||||||||||||||
| Effects | Num DF | Den DF | F value | Pr > F | Num DF | Den DF | F value | Pr > F | Num DF | Den DF | F value | Pr > F | Num DF | Den DF | F value | Pr > F | Num DF | Den DF | F value | Pr > F | |
| Colony | 2 | 23 | 0.77 | 0.4733 | 2 | 23 | 0.52 | 0.5989 | 2 | 23 | 0.54 | 0.5903 | 2 | 23 | 0.51 | 0.6052 | 2 | 23 | 1.17 | 0.3272 | |
| Sub-colony (colony) | 6 | 23 | 0.93 | 0.4892 | 6 | 23 | 0.51 | 0.7978 | 6 | 23 | 0.63 | 0.7067 | 6 | 23 | 1.67 | 0.1742 | 6 | 23 | 1.53 | 0.2127 | |
| Treatment | 1 | 23 | 2.8 | 0.1077 | 1 | 23 | 1.85 | 0.1875 | 1 | 23 | 0.6 | 0.4455 | 1 | 23 | 3.27 | 0.0838 | 1 | 23 | 1 | 0.3275 | |
| Time | 1 | 23 | 6.53 | 0.0177 | 1 | 23 | 0.88 | 0.3574 | 1 | 23 | 0.97 | 0.3361 | 1 | 23 | 0.53 | 0.4742 | 1 | 23 | 2.91 | 0.1014 | |
Table 2: Statistical results of the mixed ANOVA tests on separate behaviors for which statistical analyses could be performed. Fixed effects were colony, sub-colony (nested within colony), treatment, and time. Please click here to download this table.

Video 1: Self-grooming and antenna cleaning. Please click here to view this video. Right-click to download.

Video 2: Fungus grooming. Please click here to view this video. Right-click to download.

Video 3: Allogrooming. Please click here to view this video. Right-click to download.

Video 4: Metapleural gland grooming. Please click here to view this video. Right-click to download.

Video 5: Spore weeding. Please click here to view this video. Right-click to download.

Video 6: Fungus weeding. Please click here to view this video. Right-click to download.

Video 7: Fecal fluid grooming. Please click here to view this video. Right-click to download.

Video 8: Droplet regurgitation. Please click here to view this video. Right-click to download.
Discussion
The primary objective of this study was to observe and record characteristic leaf-cutting ant defensive behaviors in the presence of fungus-garden infection with Escovopsis, creating reference clips for use by the wider scientific community. It should be noted that these behaviors are not exclusive to defense of colonies from Escovopsis, but may also play a role in defense against other contaminants and infections6,7,8,9,10,11,12, and in the defense of the ants themselves42. Our protocol provides a backdrop for wider research on defenses in fungus-growing ants. This is likely to be particularly useful: (i) for young researchers who are not familiar with these behaviors; (ii) to secure consistent definitions for and observations of behaviors; (iii) to facilitate comparisons across studies and ant species; (iv) because a number of these behaviors may occur so infrequently that even experienced researchers may never have observed them; (iv) because understanding and recognizing behaviors in controlled conditions in the laboratory help studies in situ where conditions are harder to control.
The results from our behavioral study are consistent with previous work which showed that minor workers fungus groom — crucial if an infection is detected early — more than major workers25,28,32. Here, major workers increased the amount of fungal grooming after Escovopsis infection (Figure 2a). This suggests that minor workers are the predominant fungus groomers, but that major workers may assist in preventing the spread of more established infections. The larger major workers can remove spores faster, while minor workers could be more suited to removing less accessible spores. We also found that workers successfully removed spores in around half of the infected sub-colonies (four out of nine) when they were introduced at the time of infection, and thus could detect the pathogen early (Figure 3c). Overall, this points to a series of behavioral responses where the ants first try to stop Escovopsis infection by removing spores (and doing so before an infection spreads), rather than removing parts of the fungus garden (Figure 3a,b). This changes over time if the infection progresses, when ants are more likely to remove parts of the fungus garden28. Although our sample sizes were too small to be conclusive, and we cannot rule out that simultaneous infections induced weeding behaviors, our data supports this trend, with fungus weeding predominantly being present at later stages of infection (Figure 3a). The generally low levels of fungus weeding might suggest either that the ants used other defenses (e.g., chemicals) to inhibit further growth of Escovopsis, or that none of our experimental sub-colonies were too severely infected (making the more destructive defenses unnecessary).
Our findings suggest that self-grooming with fecal fluid is characteristic of ants entering the fungus garden, and used as a prophylactic measure, rather than being associated with an infection. Similar observations have been seen in foundress females that groom themselves and transfer fecal droplets with their mouth to their legs, when entering the nest or handling the crop27. An infection should in theory increase the activity of workers at the edge of the fungus garden, if the removed infected material is carried out and dropped in waste piles. Hence, fecal fluid grooming may also indirectly increase during infection to minimize disease spread. We would expect the opposite pattern for severe infections, with reduced movement at the edge of the fungus garden, as workers either abandon the fungus or adopt more extreme measures such as chemical defense.
While fecal fluids could serve as an important prophylactic chemical for an individual, allogrooming is used by nest-mates on other workers if they detect foreign particles or microbes. The substantial difference we observed between the frequency (Table 1) of fecal fluid grooming (n = 304) and of allogrooming (n = 48) might indicate a difference in pathogen detection. Ants are not able to easily detect pathogens on themselves with their antennae; allogrooming on the other hand is done by nest-mates, who can inspect the entire body of an ant and only choose to groom if necessary. Since Escovopsis is a parasite of the fungus garden rather than the ants, this might also explain the low amount of allogrooming.
We rarely observed metapleural gland grooming, and only at later stages of infection. Species of fungus-growing ants with abundant Pseudonocardia bacterial cover groom the metapleural glands less than species with less or no cover25,47. As A. echinatior has an abundance of the symbiont47, this may explain the low gland grooming frequency. The metapleural gland secretion is also expensive to produce30, and may be stored within the infrabuccal pocket for longer periods of time, meaning that the need for grooming of the metapleural gland may be infrequent. During metapleural gland grooming, the ants simultaneously switch legs and lick the leg that had just groomed the gland; the spores are thereby transferred to the infrabuccal pocket, where gland secretions are critical for inhibiting Escovopsis' potential for subsequent germination25. Minor workers are more abundant inside the nest and have bigger metapleural glands per unit body mass30, suggesting they are responsible for the majority of the metapleural gland secretions. This could also explain why, in our study, the highest frequency of fungus grooming was among minor workers.
We expected to observe behavior(s) indicating active use of the antibiotics from the bacterial symbiont Pseudonocardia, commonly observed on the cuticle of Acromyrmex workers and known to play a role in defense against Escovopsis36,39,40. The most likely explanation for not observing such behavior, is that the application of these antibiotics may be incorporated into other behaviors, such as self-grooming followed by fungus grooming and/or weeding, which may make it hard to observe as a distinct behavior.
We observed the unusual behavior of regurgitating liquid droplets on to the fungus garden. Regurgitation of food for nestmates has previously been described in leaf-cutting ants22. In our experiment, droplets differed in color from transparent to dark brown, suggesting they may be a food source for other ants and/or provide water. We only observed two occasions where other ants drank from the droplets, so we cannot determine if the droplets benefit other ants or serve to rehydrate the fungus when humidity is low. Most observations of this behavior were during Escovopsis infections, which might imply a defensive role, such as immune priming by regurgitation of antimicrobial peptides16,48. We cannot draw firm conclusions on this since this behavior was rare, but it would be an interesting line to investigate further, for example, by determining whether droplets have antimicrobial properties.
Given that observational studies of the complex defensive behaviors of leaf-cutter ants, including any comparison with and without fungus garden infection, would be extremely difficult to make in the field, experimental data can provide valuable insights into these behaviors under more controlled conditions. While the observations made under laboratory conditions might differ from the behaviors found under natural conditions, tools such as our catalog of key defensive behaviors need to be developed, to improve both experimental and field studies in the future. The experimental approach may, however, partially explain why some behaviors were extremely rare (e.g., allogrooming, metapleural gland grooming) in our demonstration of using these behavioral definitions. Future studies might therefore consider the limitations of this experimental setup, to find ways of making more natural observations. Additional factors could also be integrated into the current protocol, such as distinguishing between Actinobacteria-carrying (younger) workers and older workers with less abundant cover, that may respond differently to the threat of an Escovopsis infection. There are trade-offs between making observations more accurate (for example by scoring focal individuals), or having larger sub-colony size (greater number of workers), and the amount of time or number of sub-colonies or individuals that can be filmed at a given point in time. Nevertheless, while the set-up could be extended for larger behavioral studies with a focus on addressing a behavioral goal, in this case we focused on successfully showcasing a method for recording and defining specific defensive behaviors.
We documented behaviors that contribute to defense in leaf-cutting ants, and more significantly, have systematically identified, described, and captured defensive behaviors on film. Our representative results reinforce other research in this field suggesting why it is hard for a pathogen to successfully infect fungus-farming ant colonies, when facing an extensive set of defensive behaviors and associated application of antimicrobial compounds. Our main goal was to provide a new tool for future work in this field, and we hope that the behavioral catalog will prove valuable for securing consensus and streamlined definitions, observations, and interpretations of behaviors, to serve as an important resource for future research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Line Vej Ugelvig and Kirsten Sheehy for useful discussions regarding grooming mechanisms of the antenna cleaners and filming set-up. Michael Poulsen is supported by a Villum Kann Rasmussen Young Investigator Fellowship (VKR10101) and Tabitha Innocent by a Marie Curie Individual European fellowship (IEF grant 627949). We would also like to thank the Smithsonian Tropical Research Institute for the use of facilities and logistical support in Panama, and the Autoridad Nacional del Ambiente for permission to collect and export ants to Denmark.
Materials
| Name | Company | Catalog Number | Comments |
| Plastic boxes | N/A | N/A | Transparent. Length: 3.15 in (8 cm), width: 2.17 in (5.5 cm), depth: 1.77 in (4.5 cm) |
| Petri dishes | Sarstedt | 82.1472 | 3.62x0.63 in (9.2x1.6 cm) |
| Inoculation loops | Labsolute | 7696431 | Disposable 1uL. Length: 7.67 in (19.5 cm) |
| Cameras | DBPower | NTC50HD_8.5mm | USB Endoscope Camera |
| Holders for the cameras | N/A | N/A | Old beaker clamp stand. |
| Laptop | HP | N/A | Generic laptop for saving recordings. |
| Program used on the laptop | Windows Movie maker | N/A | |
| Forceps | Vermandel | 50.054 | Soft |
| Potato dextrose broth | Sigma-Aldrich | P6685-1KG | 24 g/L |
References
- Hölldobler, B., Wilson, E. O. . The ants. , (1990).
- Weber, N. A. . Gardening ants, the attines. , (1972).
- Bot, A. N. M., Ortius-Lechner, D., Finster, K., Maile, R., Boomsma, J. J. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insectes Sociaux. 49 (4), 363-370 (2002).
- Poulsen, M., Boomsma, J. J. Mutualistic fungi control crop diversity in fungus-growing ants. Science. 307 (5710), 741-744 (2005).
- Mueller, U. G., Scott, J. J., Ishak, H. D., Cooper, M., Rodrigues, A. Monoculture of Leafcutter Ant Gardens. Plos One. 5 (9), (2010).
- Hughes, W. O. H., Thomsen, L., Eilenberg, J., Boomsma, J. J. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. Journal of Invertebrate Pathology. 85 (1), 46-53 (2004).
- Samson, R. A., Evans, H. C., Latgé, J. -. P. . Atlas of entomopathogenic fungi. , (1988).
- Shah, P. A., Pell, J. K. Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology. 61 (5-6), 413-423 (2003).
- Hughes, D. P., Evans, H. C., Hywel-Jones, N., Boomsma, J. J., Armitage, S. A. O. Novel fungal disease in complex leaf-cutting ant societies. Ecological Entomology. 34 (2), 214-220 (2009).
- Andersen, S. B., et al. The Life of a Dead Ant: The Expression of an Adaptive Extended Phenotype. American Naturalist. 174 (3), 424-433 (2009).
- Currie, C. R., Mueller, U. G., Malloch, D. The agricultural pathology of ant fungus gardens. Proceedings of the National Academy of Sciences of the United States of America. 96 (14), 7998-8002 (1999).
- Reynolds, H. T., Currie, C. R. Pathogenicity of Escovopsis weberi: The parasite of the attine ant-microbe symbiosis directly consumes the ant-cultivated fungus. Mycologia. 96 (5), 955-959 (2004).
- Camargo, R. S., Forti, L. C., Lopes, J. F. S., Andrade, A. P. P., Ottati, A. L. T. Age polyethism in the leaf-cutting ant Acromyrmex subterraneus brunneus Forel, 1911 (Hym., Formicidae). Journal of Applied Entomology. 131 (2), 139-145 (2007).
- Hughes, W. O. H., Sumner, S., Van Borm, S., Boomsma, J. J. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proceedings of the National Academy of Sciences of the United States of America. 100 (16), 9394-9397 (2003).
- Wilson, E. O. Caste and Division of Labor in Leaf-Cutter Ants (Hymenoptera, Formicidae, Atta). 2. The Ergonomic Optimization of Leaf Cutting. Behavioral Ecology and Sociobiology. 7 (2), 157-165 (1980).
- Schmid-Hempel, P. Parasites and Their Social Hosts. Trends in Parasitology. 33 (6), 453-462 (2017).
- Hamilton, W. D., Axelrod, R., Tanese, R. Sexual Reproduction as an Adaptation to Resist Parasites (a Review). Proceedings of the National Academy of Sciences of the United States of America. 87 (9), 3566-3573 (1990).
- Zhu, Y. Y., et al. Genetic diversity and disease control in rice. Nature. 406 (6797), 718-722 (2000).
- Heine, D., et al. Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen. Nature Communications. 9, (2018).
- Cremer, S., Pull, C. D., Furst, M. A. Social Immunity: Emergence and Evolution of Colony-Level Disease Protection. Annual Review of Entomology. 63, 105-123 (2018).
- Quinlan, R. J., Cherrett, J. M. Role of Substrate Preparation in Symbiosis between Leaf-Cutting Ant Acromyrmex octospinosus (Reich) and Its Food Fungus. Ecological Entomology. 2 (2), 161-170 (1977).
- Richard, F. J., Errard, C. Hygienic behavior, liquid-foraging, and trophallaxis in the leaf-cutting ants, Acromyrmex subterraneus and Acromyrmex octospinosus. Journal of Insect Science. 9, (2009).
- Reber, A., Purcell, J., Buechel, S. D., Buri, P., Chapuisat, M. The expression and impact of antifungal grooming in ants. Journal of Evolutionary Biology. 24 (5), 954-964 (2011).
- Poulsen, M., Bot, A. N. M., Boomsma, J. J. The effect of metapleural gland secretion on the growth of a mutualistic bacterium on the cuticle of leaf-cutting ants. Naturwissenschaften. 90 (9), 406-409 (2003).
- Fernandez-Marin, H., Zimmerman, J. K., Rehner, S. A., Wcislo, W. T. Active use of the metapleural glands by ants in controlling fungal infection. Proceedings of the Royal Society B-Biological Sciences. 273 (1594), 1689-1695 (2006).
- Fernandez-Marin, H., et al. Functional role of phenylacetic acid from metapleural gland secretions in controlling fungal pathogens in evolutionarily derived leaf-cutting ants. Proceedings of the Royal Society B-Biological Sciences. 282 (1807), (2015).
- Fernandez-Marin, H., Zimmermann, J. K., Wcislo, W. T. Nest-founding in Acromyrmexoctospinosus (Hymenoptera, Formicidae, Attini): demography and putative prophylactic behaviors. Insectes Sociaux. 50 (4), 304-308 (2003).
- Currie, C. R., Stuart, A. E. Weeding and grooming of pathogens in agriculture by ants. Proceedings of the Royal Society B-Biological Sciences. 268 (1471), 1033-1039 (2001).
- Ortius-Lechner, D., Maile, R., Morgan, E. D., Boomsma, J. J. Metaplural gland secretion of the leaf-cutter ant Acromyrmex octospinosus: New compounds and their functional significance. Journal of Chemical Ecology. 26 (7), 1667-1683 (2000).
- Poulsen, M., Bot, A. N. M., Nielsen, M. G., Boomsma, J. J. Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behavioral Ecology and Sociobiology. 52 (2), 151-157 (2002).
- Bot, A. N. M., Currie, C. R., Hart, A. G., Boomsma, J. Waste management in leaf-cutting ants. Ethology Ecology & Evolution. 13 (3), 225-237 (2001).
- Little, A. E. F., Murakami, T., Mueller, U. G., Currie, C. R. Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus gardens. Biology Letters. 2 (1), 12-16 (2006).
- Abramowski, D., Currie, C. R., Poulsen, M. Caste specialization in behavioral defenses against fungus garden parasites in Acromyrmex octospinosus leaf-cutting ants. Insectes Sociaux. 58 (1), 65-75 (2011).
- Currie, C. R., Scott, J. A., Summerbell, R. C., Malloch, D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 398 (6729), 701-704 (1999).
- Scheuring, I., Yu, D. W. How to assemble a beneficial microbiome in three easy steps. Ecology Letters. 15 (11), 1300-1307 (2012).
- Worsley, S. F., et al. Symbiotic partnerships and their chemical interactions in the leafcutter ants (Hymenoptera: Formicidae). Myrmecological News. 27, 59-74 (2018).
- Currie, C. R., Poulsen, M., Mendenhall, J., Boomsma, J. J., Billen, J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science. 311 (5757), 81-83 (2006).
- Poulsen, M., et al. Variation in Pseudonocardia antibiotic defence helps govern parasite-induced morbidity in Acromyrmex leaf-cutting ants. Environmental Microbiology Reports. 2 (4), 534-540 (2010).
- Poulsen, M., Cafaro, M., Boomsma, J. J., Currie, C. R. Specificity of the mutualistic association between actinomycete bacteria and two sympatric species of Acromyrmex leaf-cutting ants. Molecular Ecology. 14 (11), 3597-3604 (2005).
- Andersen, S. B., Hansen, L. H., Sapountzis, P., Sorensen, S. J., Boomsma, J. J. Specificity and stability of the Acromyrmex-Pseudonocardia symbiosis. Molecular Ecology. 22 (16), 4307-4321 (2013).
- Currie, C. R., Bot, A. N. M., Boomsma, J. J. Experimental evidence of a tripartite mutualism: bacteria protect ant fungus gardens from specialized parasites. Oikos. 101 (1), 91-102 (2003).
- Cremer, S., Armitage, S. A. O., Schmid-Hempel, P. Social immunity. Current Biology. 17 (16), R693-R702 (2007).
- Schluns, H., Crozier, R. H. Molecular and chemical immune defenses in ants (Hymenoptera: Formicidae). Myrmecological News. 12, 237-249 (2009).
- Masri, L., Cremer, S. Individual and social immunisation in insects. Trends in Immunology. 35 (10), 471-482 (2014).
- Hackmann, A., Delacave, H., Robinson, A., Labonte, D., Federle, W. Functional morphology and efficiency of the antenna cleaner in Camponotus rufifemur ants. Royal Society Open Science. 2 (7), (2015).
- . How ants use 'combs' and 'brushes' to keep their antennae clean Available from: https://www.youtube.com/watch?time_continue=65&v=AB4HoeloqZw (2015)
- Fernandez-Marin, H., Zimmerman, J. K., Nash, D. R., Boomsma, J. J., Wcislo, W. T. Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proceedings of the Royal Society B-Biological Sciences. 276 (1665), 2263-2269 (2009).
- Konrad, M., et al. Social Transfer of Pathogenic Fungus Promotes Active Immunisation in Ant Colonies. Plos Biology. 10 (4), (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved