Method Article
Activity-based Training on a Treadmill with Spinal Cord Injured Wistar Rats
In This Article
Summary
This protocol demonstrates our model of activity-based locomotor treadmill training for rats with spinal cord injury (SCI). Included is both quadrupedal and forelimb-only groups, in addition to two distinct types of non-trained control groups. Investigators are able to assess training effects on SCI rats using this protocol.
Abstract
Spinal cord injury (SCI) results in lasting deficits that include both mobility and a multitude of autonomic-related dysfunctions. Locomotor training (LT) on a treadmill is widely used as a rehabilitation tool in the SCI population with many benefits and improvements to daily life. We utilize this method of activity-based task-specific training (ABT) in rodents after SCI to both elucidate the mechanisms behind such improvements and to enhance and improve upon existing clinical rehabilitation protocols. Our current goal is to determine the mechanisms underlying ABT-induced improvements in urinary, bowel, and sexual function in SCI rats after a moderate to severe level of contusion. After securing each individual animal in a custom-made adjustable vest, they are secured to a versatile body weight support mechanism, lowered to a modified three-lane treadmill and assisted in step-training for 58 minutes, once a day for 10 weeks. This setup allows for the training of both quadrupedal and forelimb-only animals, alongside two different non-trained groups. Quadrupedal-trained animals with body weight support are aided by a technician present to assist in stepping with proper hind limb placement as necessary, while forelimb-only trained animals are raised at the caudal end to ensure no hind limb contact with the treadmill and no weight-bearing. One non-trained SCI group of animals is placed in a harness and rests next to the treadmill, while the other control SCI group remains in its home cage in the training room nearby. This paradigm allows for the training of multiple SCI animals at once, thus making it more time-efficient in addition to ensuring that our pre-clinical animal model mimics the clinical representation as close as possible, particularly with respect to the body weight support with manual assistance.
Introduction
Globally, between 250,000 - 500,000 new spinal cord injury (SCI) cases arise either due to degeneration, diseases, or most commonly (up to 90%) trauma1. After traumatic SCI, a series of physiologic events take place that result in neurological deficits that affect a multitude of bodily functions. Due to the chronic deficits that follow SCI, the development and testing of effective treatment modalities is crucial. Until recently, rehabilitation strategies have most commonly focused on recovery of mobility2,3. Following SCI, patients rank bladder/urinary, bowel, and sexual functions among the highest quality of life complications in need of better management1,4,5. Therefore, targeting bladder, bowel, and sexual function is of utmost importance from a rehabilitation standpoint1,4,5.
Exercise and locomotor training (LT) are commonly utilized rehabilitative therapies in the SCI patient population with many benefits such as cardiovascular function, bladder/urinary function, and mobility6,7,8,9,10. It is for this reason we utilize a similar modality in our pre-clinical rat SCI model. It is our goal to determine what effects LT has on SCI Wistar rats, specifically regarding both upper (kidney) and lower (bladder, external urethral sphincter) urinary tract function, bowel function, and sexual function. Further, LT has been shown to be sufficient in activating neuromuscular systems below the level of injury which may influence the amount of plasticity within the central nervous system (CNS)11,12.
The success of LT in pre-clinical studies is well documented in both large13,14 and small15,16,17,18,19 SCI animal models. Evidence suggests that afferent sensory input provided by LT is sufficient to stimulate spinal reflex pathways that result in plasticity and improvements to sensory-motor function9,20. LT benefits regarding autonomic functions have not been well characterized. For this reason, we implement our training paradigm with a focus on autonomic outcome measures, using four distinct groups that include two non-trained controls and a metabolic/exercise non- weight bearing group alongside an LT group that mimics the timing, session duration, manual assistance and weight support that are used in clinical studies19,21,22,23,24.
Protocol
All methods described have been approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC).
1. Pre-injury Handling and Testing (One Week Before SCI)
- Handle each rat for a period of 5 - 10 min once a day for five days.
NOTE: Adult male Wistar rats that are ~50 days of age initially and weigh 200 - 225 g are used in this protocol. Rats at this pre-injury time-point are not acclimated to the harness that is used for LT as full use of hindlimbs allows the rat to escape from the jacket. - Conduct any pre-injury testing that is study-specific (e.g., the authors do metabolic cage assessments for studies that involve the effects of SCI on bladder and bowel function).
2. Spinal Cord Contusion25,26,27,28
- Anesthetize animals with ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture intraperitoneally according to provided dosage chart (Table 1). Administer supplemental dosing as needed. Test anesthetic depth at least every 10 min by assessing corneal, palpebral, pedal, tail pinch, and pinna reflexes.
- Shave hair from the back of the animal where incision and injury are to occur. Cleanse the surgical area with Dermachlor 4% Surgical scrub. Administer a long-acting general antibiotic (e.g., 0.5 cc Pro-Pen-G subcutaneously).
- Place the anesthetized animal on a heating pad at a low setting to maintain normal body temperature.
- Estimate location of targeted lesion level based upon vertebral protuberances and with a #10 scalpel, make an estimated 5 cm incision on the dorsum of the animal, directly above the midline vertebrae.
- For mid-thoracic contusions, expose the T8/T9 level of spinal cord via removal (with rongeurs) of the overlaying T7 vertebral lamina.
- Using a contusion device such as an infinite horizon impactor29, perform the contusion (for a moderate to severe degree of SCI, use a force of 210 kdyn with no dwell time)18.
- Suture together the muscular layer and fascia over the spinal cord using 4-0 diameter monofilament and close the skin with 9 mm surgical wound clips.
- Administer postoperative drugs, such as gentamicin sulfate (5 mg/kg per day for 5 days; antibiotic to avoid bladder infections) and meloxicam (1 mg/kg subcutaneously, analgesic for first 48 h and then as needed).
- Place animals in a clean cage on a heating pad. Check animal vital signs every 15 minutes until they are fully awake from anesthesia. During the first post-op day, the animals are encouraged to eat with a sugary treat. For the first 48 h (three times daily at the time of manual crede - see 2.10), rats are monitored for inactivity, vocalization in response to handling, and lack of desire to eat and drink. If analgesia is found to be inadequate, the veterinary staff is contacted. Throughout the initial two-week recovery phase, the animals are observed for evidence of infection or other complications. Once reflex voiding returns, the animals are tended to twice a day (early morning and late afternoon). Animals with infections or significant weight loss are immediately euthanized. Regarding food and water intake, the cut off point for euthanasia is when the animal has reached anything over 20% weight loss. Normal weight loss after surgery and disuse atrophy of muscles below the level of injury is 15-20%. All animals are weighed at least once per week.
- Perform bladder emptying procedures using the manual Credé maneuver 3 times a day (8 am, 3 pm, 10 pm) until reflexive bladder function has returned (3 - 6 days on average for contusions)26,30.
3. Training Phase
- Commence LT no earlier than two weeks post-SCI, as initiating interventions too early may exacerbate secondary injury cascades31.
- Week 1 acclimation to treadmill training: Transport the rats to a quiet room that is dedicated for training.
- On Day 1, randomly and evenly divide the SCI animals into trained and non-trained control groups, to account for potential variability in both the injury itself as well as the degree of spontaneous recovery after contusion. For example, divide rats into 4 separate groups: quadrupedal trained (QT), forelimb-only trained (FT), non-trained control (NT), and non-trained home cage control (HC). A sham group where animals receive a laminectomy but no injury and are otherwise handled the same as the other groups can also be used as an uninjured control group without training.
- Place each animal in the respective harness (Figure 1) and fasten harnesses to the body weight support mechanism above the treadmill via alligator clips which are fastened to weight support springs (Figure 2 and Figure 3). This requires the animal to be fixed in one spot on the treadmill, ensuring that they go in the designated forward direction and speed.
NOTE: Due to time and personnel constraints, the authors’ lab conducts daily training in groups of twelve animals, three in each subset group. - Start the acclimation process following the previously published protocol17. Commence acclimation to LT (start of week 3 post-SCI) with a gradual treadmill exposure regimen, increasing from 10 min on Day 1 to the full target of 58 min over the first week (Table 2). Typically, by Day 4, the animals acclimate well to the training regimen. If an animal does not show progression by the third day of acclimation, the time would be reduced, and extra days added at a more gradual ramp-up (rare occurrence).
- If an animal during the first day or two does not adapt to the confinement of the harness and treadmill, stop the training session, remove it from the harness, place the animal back in its cage, and give it two treats to help reinforce future compliance. The next day, place the animal in the harness and weight support system again for 10 min. On subsequent days, increase the duration by 20 min initially then continue to increase the training duration daily to achieve full training by Day 10.
- Follow the detailed training regimen provided in Table 2.
- Due to limited hind limb use post-injury, rats in the QT group will require manual facilitation for proper paw placement while stepping on the treadmill. Use one finger on each hand (commonly the third digit) to aid in hip/waist support. When the animal requires further assistance in stepping, use this same finger to apply pressure above the knee to initiate stepping. If necessary, use a separate finger (commonly the fifth digit) to aid the foot in stepping.
NOTE: The amount of body weight support needed varies from animal to animal and changes as training progresses. The spring support system gives enough assistance to keep the animal positioned for a proper gait. Further support is provided as needed by the trainer per above. Note that a key element of LT is functionally appropriate paw placement for stepping and interlimb coordination that is promoted by the trainer and is independent of the support system. - For the FT exercise group, adjust the body weight support system to slightly elevate the hind limbs to ensure no sensory stimuli to the paws and no weight bearing is occurring through contact with the treadmill.
NOTE: The FT group serves as an exercise and metabolic control, similar to that of a hand-crank exercise in human activity-based training studies. - Have the NT group harnessed and attached to the body weight support system in a similar fashion as the QT and place the NT group near the QT group on a stationary surface (Figure 2 and Figure 3).
NOTE: The NT group receives no activity and controls for any potential effects of being harnessed for an extended time period. - A home cage group can serve as an additional control. Transport these animals to the training facility as an additional step for this group.
- Due to limited hind limb use post-injury, rats in the QT group will require manual facilitation for proper paw placement while stepping on the treadmill. Use one finger on each hand (commonly the third digit) to aid in hip/waist support. When the animal requires further assistance in stepping, use this same finger to apply pressure above the knee to initiate stepping. If necessary, use a separate finger (commonly the fifth digit) to aid the foot in stepping.
- By Day 7 -10 following the start of LT, train each animal once daily, every day until the day of termination of the study. Following each day of training, give each animal a sugary treat to reinforce compliance. Continue daily LT on animals following the 1 h regimen provided in Table 2 for the duration of the study (e.g., 8-12 weeks to mimic the approximate 80 one-hour sessions that are done in clinical studies)9.
4. Euthanasia and Tissue Collection
- Administer a lethal dose of anesthesia to the animal that adheres to AVMA guidelines on Euthanasia.
- When the heart is just barely beating, immediately begin perfusing the animal in a dedicated fume hood first with cold heparinized saline followed by cold, 4% paraformaldehyde solution.
- Begin by using surgical scissors to make an incision across the diaphragm, exposing the thoracic cavity. Continue to cut through the ribcage rostrally on both sides, removing the ribcage. Insert the perfusion needle into the left ventricle of the heart and clamp needle with hemostats, then clip the right atrium.
- Using a perfusion pump mechanism, allow the cold heparinized saline to flow through the animal’s blood vessels. Once clear saline flows from the right atrium, switch over to the cold 4% paraformaldehyde solution, until the body has stiffened.
- Remove necessary tissue such as kidney, bladder, colon, brain, sensory ganglia, and spinal cord, and store in 4% paraformaldehyde for up to 48 h at 4 °C. After 24 - 48 h, move tissue to 30% sucrose and store at 4 °C.
- Move collected tissue to a 30% sucrose/phosphate buffered cryoprotectant solution until tissue is ready for cutting. To cut tissue, embed in a tissue freezing compound and cut on a cryostat at desired thickness depending on the type of tissue used (e.g., 35 µm for brain and spinal cord tissue, 5 - 7 µm for organ tissues).
Results
Following this training protocol, it has been documented that only the QT animals demonstrate superior locomotor function when compared to the other groups18. However, due to the nature of our lab, our primary focus is to investigate non-locomotor benefits of activity-based task-specific training (ABT), including bladder, bowel, and sexual function. For instance, we have previously published data that shows LT results in an exercise-induced reduction of polyuria in both QT and FT groups of SCI rats (Figure 4)17. Also, an injury-induced decrease in transforming growth factor-β (TGF-β) expression in the kidney's, indicative of an altered immune response, was not seen in QT and FT groups, which had TGF-β levels similar to sham (no injury) animals. In the same study17, awake cystometry was performed before euthanasia and tissue collection. The maximum amplitude of bladder contractions during void cycles was not significantly different across sham, QT, and FT groups, while NT groups remained significantly altered. Together, these data indicate a positive exercise outcome on kidney health and bladder function, thus improving urinary function after SCI.
The mechanisms underlying polyuria within the SCI population is currently not clear but is likely multi-factorial32. Some have hypothesized, for example, that pooling of fluid in the lower limbs while SCI individuals are in a wheelchair can lead to fluid overload and increased fluid elimination during postural shifts (such as moving from sitting to lying)33. Such an explanation does not hold for the pre-clinical model, which has led us to focus initially on arginine vasopressin (AVP), the hormone which controls fluid homeostasis in the body and can be modulated with exercise. AVP controls fluid homeostasis through activation of the V2 receptor in the kidneys which facilitates water resorption from the renal collecting ducts34. Preliminary evidence from a pilot experiment (chronic time-point with one lesion severity - 210 kdyn impact force) indicate a beneficial effect of exercise (LT and FT) on V2 receptor levels in the rat kidney (Figure 5).
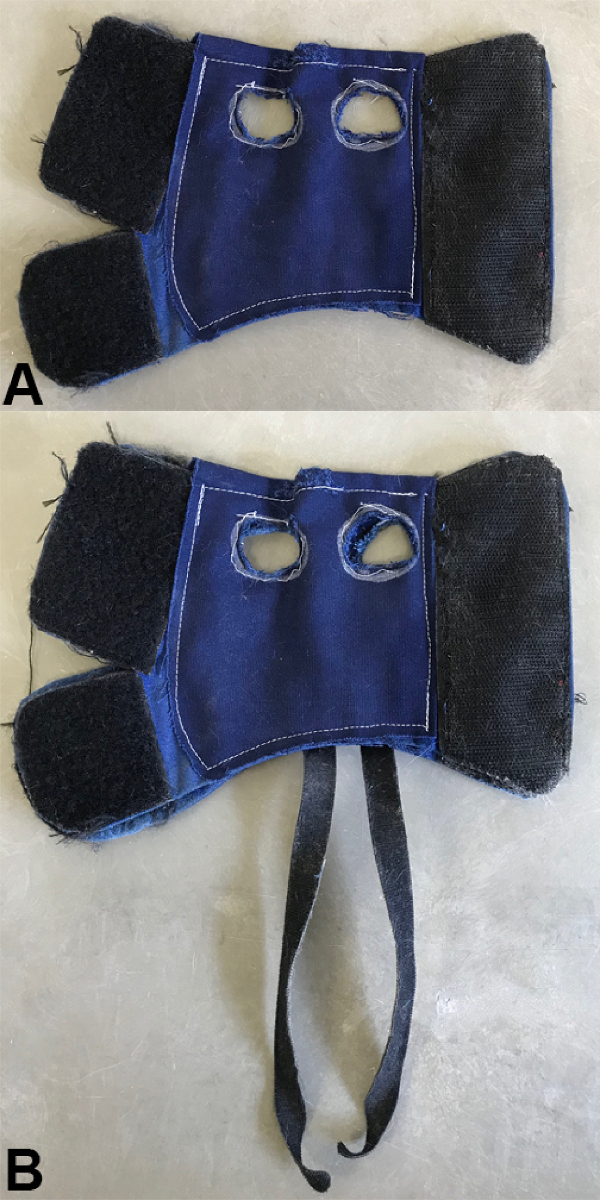
Figure 1: Custom-made harnesses sized for male Wistar rats. Both QT and NT animals are placed in the same type of jacket (A) allowing for the use of hind limbs in the case of QT animals. There are additional straps sewed onto the harness used for FT animals (B) to raise up the hind limbs, assuring no body weight support. The large hook-and-loop material portions of the harness allow for easy adjustments to different sized animals and to any changes in the size of an individual animal over time. Please click here to view a larger version of this figure.
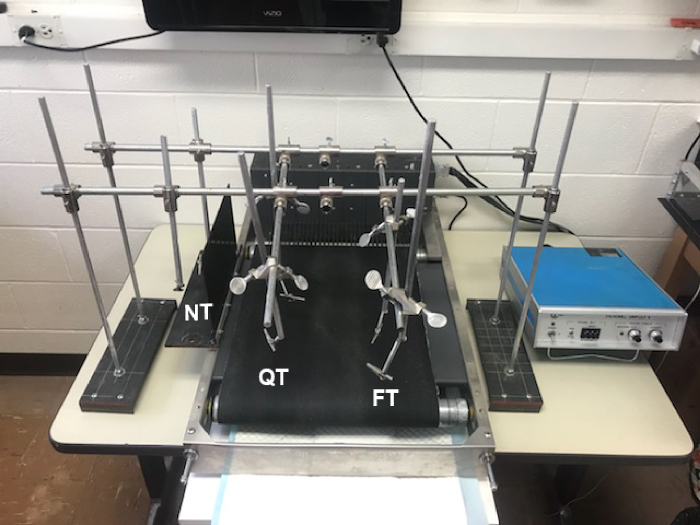
Figure 2: Training station setup. Body weight support mechanism surrounding the treadmill for either NT (far left), QT (middle), or FT (right) groups. Please click here to view a larger version of this figure.
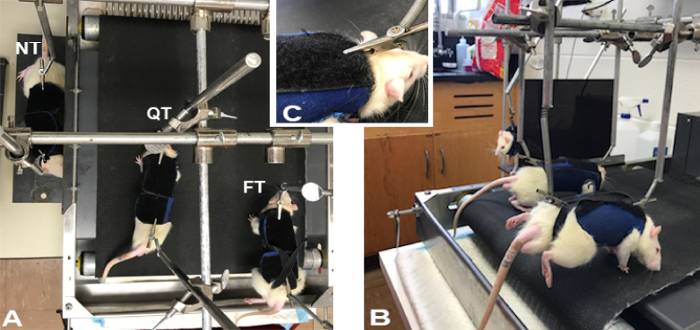
Figure 3: Training station with animals. Top (A) and (B) side views showing body weight support mechanism and location of attachment support clips to the harnesses. Note that the hind limbs of the FT animal (B) is raised and off the treadmill belt. Inset (C) portrays a closer view of the clip fastened to the harness. Please click here to view a larger version of this figure.
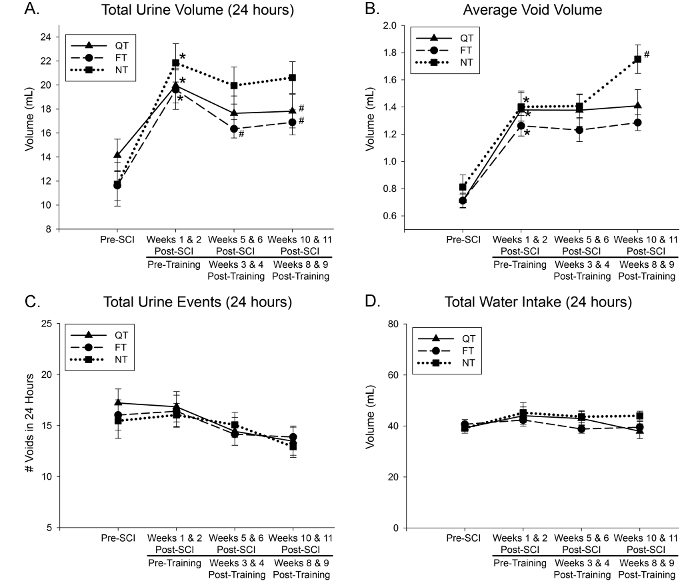
Figure 4: ABT effects on rat polyuria after SCI. The total volume of urine output (A) increased after SCI (*; p < 0.05) and returned closer to baseline after 9 weeks of LT training in both QT and FT groups but remained increased in the NT group relative to the trained groups (#; p < 0.05). All groups demonstrated increased urine output compared to baseline at 9 weeks and increased void volume (B). It is important to note that the number of voids (C) and the amount of water intake (D) remained the same across all groups. Values are means ± standard error. This figure is republished with author permission17. Please click here to view a larger version of this figure.

Figure 5: ABT effects on rat kidney. Western blot results for rat kidney levels of V2 receptors in 5 groups of 4 rats each (20 total), showing expression levels for the protein bands provided in panel A and group mean densitometry analysis results of the bands (using ImageJ; OD = optical density) in panel B, indicating a significant (*; p < 0.05) decrease in receptors at a chronic time-point (12 weeks) post-SCI and no decrease relative to baseline (sham surgical controls) for groups receiving 10 weeks of one-hour daily ABT. Error bars represent standard error. Please click here to view a larger version of this figure.
| Ketamine/Xylazine Dose Chart | ||||
| Effective dose: | ***Using 100 mg/mL ketamine stock and 20 mg/mL xylazine stock*** | |||
| 80 mg/kg ketamine | ||||
| 10 mg/kg xylazine | ||||
| 1.0 mL mixture Injection = 0.62 mL ketamine stock (100 mg/mL) + 0.38 mL xylazine stock (20 mg/mL) | ||||
| Animal Weight | Mixture Injection | Animal Weight | Mixture Injection | |
| (g) | (mL) | (g) | (mL) | |
| 100 | 0.13 | 275 | 0.36 | |
| 105 | 0.14 | 285 | 0.37 | |
| 110 | 0.14 | 290 | 0.38 | |
| 115 | 0.15 | 300 | 0.39 | |
| 120 | 0.16 | 305 | 0.4 | |
| 125 | 0.16 | 310 | 0.4 | |
| 130 | 0.17 | 315 | 0.41 | |
| 135 | 0.18 | 320 | 0.42 | |
| 140 | 0.18 | 325 | 0.42 | |
| 145 | 0.19 | 330 | 0.43 | |
| 150 | 0.2 | 335 | 0.44 | |
| 155 | 0.2 | 340 | 0.44 | |
| 160 | 0.21 | 345 | 0.45 | |
| 165 | 0.21 | 350 | 0.46 | |
| 170 | 0.22 | 355 | 0.46 | |
| 175 | 0.23 | 360 | 0.47 | |
| 180 | 0.23 | 365 | 0.47 | |
| 185 | 0.24 | 370 | 0.48 | |
| 190 | 0.25 | 375 | 0.49 | |
| 195 | 0.25 | 380 | 0.49 | |
| 200 | 0.26 | 385 | 0.5 | |
| 205 | 0.27 | 390 | 0.51 | |
| 210 | 0.27 | 395 | 0.51 | |
| 215 | 0.28 | 400 | 0.52 | |
| 220 | 0.29 | 410 | 0.53 | |
| 225 | 0.29 | 420 | 0.55 | |
| 230 | 0.3 | 430 | 0.56 | |
| 235 | 0.31 | 440 | 0.57 | |
| 240 | 0.31 | 450 | 0.59 | |
| 245 | 0.32 | 460 | 0.6 | |
| 250 | 0.33 | 470 | 0.61 | |
| 255 | 0.33 | 480 | 0.62 | |
| 260 | 0.34 | 490 | 0.64 | |
| 265 | 0.34 | 500 | 0.65 | |
| 270 | 0.35 | 510 | 0.66 | |
Table 1: Anesthesia dosage chart based upon individual animal's weight.
| Training Time (min) | Speed (cm/s) | Duration (min) |
| 0-1 | 6 | 1 |
| 1-2 | 8.4 | 1 |
| 2-3 | 10.8 | 1 |
| 3-8 | 13.2 | 5 |
| 8-13 | 10.8 | 5 |
| 13-28 | 13.2 | 15 |
| 28-33 | 10.8 | 5 |
| 33-38 | 6 | 5 |
| 38-43 | 8.4 | 5 |
| 43-58 | 13.2 | 15 |
Table 2: Training regimen of speed settings the treadmill should be on corresponding to the time spent at each speed.
Discussion
Our methods of ABT on rats after SCI is a novel therapeutic intervention. While other methods of exercise and step training in animal models may exist35,36,37, this method mimics LT carried out clinically in the SCI human population, where we have seen promising results23. With the combination of our setup, regimen, and use of control animals, the results obtained from utilizing our training paradigm will help to understand the benefits of ABT after SCI. Future applications of this protocol include observing the described outcomes of ABT at different training timeframes as well as the effect that ABT has on recovery from different levels and extents of injury.
One limitation of this design is the length of time for such experiments. Given that our training regimen for each animal requires 1 hour per day, every day for 10 weeks, substantial personnel time and an organized schedule is a necessity. An important aspect that requires special attention involves the FT group, which has unique harnesses with hook-and-loop material straps to secure the hind limbs above the treadmill for the elimination of weight support. It is important to ensure that the animal does not receive weight support, which is why a platform is not positioned under the rat's hind paws. In addition, as previous studies have indicated that the sensory input is a principal driver of locomotor system plasticity in the spinal cord38,39,40, there is a constant need of handling the QT group to assist with stepping much the same as physical therapists in the clinical setting.
An important modification made to the commercially available treadmill system used for the animals was reversing the polarity. After exposing the motor, the positive and negative wires were switched which reverses the direction the treadmill moves. This allows for more space and easier access to reach and help train the animals (the system comes with a shock grid at one end that is designed to prevent non-harnessed, spinally intact animals from stepping off the treadmill belt).
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Drs. Patricia Ward, April Herrity and Susan Harkema for their input and guidance, Christine Yarberry for surgical assistance, Yangsheng Chen, Andrea Willhite and Johnny Morehouse for technical assistance and Darlene Burke for assistance with statistics and behavioral assessments. Funding support for this work was provided by the Department of Defense (W81XWH-11-1-0668 and W81XWH-15-1-0656) and the Kentucky Spinal Cord and Head Injury Research Trust (KSCHIRT 14-5).
Materials
| Name | Company | Catalog Number | Comments |
| Exer-3R treadmill | Columbus Instruments | reversed polarity of the motor | |
| Body weight support system | N/A | N/A | modified spring scales with alligator clips |
| Rat harness | N/A | N/A | Our harnesses are custom made; please refer to Figure 1 for visual. |
| Infinite Horizon (IH) impactor device | Precision Systems and Instrumentation | Model 0400 | |
| Ketamine HCl | Hospira | NDC 0409-2053-10 | |
| Xylazine (AnaSed Injection) | Akorn Animal Health | NDC 59399-110-20 | |
| Meloxicam (Eloxiject) | Henry Schein Animal Health | NDC 116695-6925-2 | |
| Gentamicin Sulfate (GentaFuse) | Henry Schein Animal Health | NDC 11695-4146-1 | |
| urethane, 97% | Argos Organics | CAS 51-79-6 | |
| 4-0 monofilament suture kit (4-0 Ethilon Nylon Suture) | Ethicon, LLC | 205016 | |
| Michel suture clips (9mm Auto Clips) | MikRon Precision, Inc. | 1629 | |
| Heating pad | Mastex Industries, Inc | Model 500 | |
| Tootie Fruitys cereal | Malt O Meal | For training reward | |
| Male Wistar rats | Envigo | ||
| Size 10 surgical scalpel blades | Miltex | SKU: 4-110 |
References
- Ahuja, C. S., et al. Traumatic spinal cord injury. Nature Reviews Disease Primers. 3, 17018 (2017).
- Behrman, A. L., Harkema, S. J. Locomotor training after human spinal cord injury: a series of case studies. Physical Therapy. 80 (7), 688-700 (2000).
- Anderson, K. D. Targeting recovery: priorities of the spinal cord-injured population. Journal of Neurotrauma. 21 (10), 1371-1383 (2004).
- Steadman, C. J., Hubscher, C. H. Sexual function after spinal cord injury: innervation, assessment, and treatment. Current Sexual Health Reports. 8 (2), 106-115 (2016).
- Behrman, A. L., et al. Locomotor training progression and outcomes after incomplete spinal cord injury. Physical Therapy. 85 (12), 1356-1371 (2005).
- Dietz, V., Harkema, S. J. Locomotor activity in spinal cord-injured persons. Journal of Applied Physiology. 96 (5), 1954-1960 (2004).
- Harkema, S., et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. The Lancet. 377 (9781), 1938-1947 (2011).
- Harkema, S. J., et al. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Archives of physical medicine and rehabilitation. 93 (9), 1588-1597 (2012).
- Jayaraman, A., et al. Locomotor training and muscle function after incomplete spinal cord injury: case series. The Journal of Spinal Cord Medicine. 31 (2), 185-193 (2008).
- Behrman, A. L., Bowden, M. G., Nair, P. M. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Physical Therapy. 86 (10), 1406-1425 (2006).
- Edgerton, V. R., Tillakaratne, N. J., Bigbee, A. J., de Leon, R. D., Roy, R. R. Plasticity of the spinal neural circuitry after injury. Annual Review of Neuroscience. 27, 145-167 (2004).
- Barbeau, H., Rossignol, S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Research. 412 (1), 84-95 (1987).
- Lovely, R. G., Gregor, R., Roy, R., Edgerton, V. R. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Experimental Neurology. 92 (2), 421-435 (1986).
- Multon, S., Franzen, R., Poirrier, A. -. L., Scholtes, F., Schoenen, J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. Journal of Neurotrauma. 20 (8), 699-706 (2003).
- Moraud, E. M., et al. Closed-loop control of trunk posture improves locomotion through the regulation of leg proprioceptive feedback after spinal cord injury. Scientific Reports. 8 (1), 76 (2018).
- Hubscher, C. H., et al. Effects of exercise training on urinary tract function after spinal cord injury. American Journal of Physiology-Renal Physiology. 310 (11), F1258-F1268 (2016).
- Ward, P. J., et al. Novel multi-system functional gains via task specific training in spinal cord injured male rats. Journal of Neurotrauma. 31 (9), 819-833 (2014).
- Ward, P. J., et al. Optically-induced neuronal activity is sufficient to promote functional motor axon regeneration in vivo. PloS One. 11 (5), e0154243 (2016).
- Edgerton, V. R., et al. Retraining the injured spinal cord. The Journal of physiology. 533 (1), 15-22 (2001).
- Angeli, C. A., Edgerton, V. R., Gerasimenko, Y. P., Harkema, S. J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 137 (5), 1394-1409 (2014).
- Behrman, A. L., Ardolino, E. M., Harkema, S. J. Activity-Based Therapy: From basic science to clinical application for recovery after spinal cord injury. Journal of Neurologic Physical Therapy. 41, S39-S45 (2017).
- Hubscher, C. H., et al. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PloS One. 13 (1), e0190998 (2018).
- Rejc, E., Angeli, C. A., Bryant, N., Harkema, S. J. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. Journal of Neurotrauma. 34 (9), 1787-1802 (2017).
- Hall, B. J., et al. Spinal cord injuries containing asymmetrical damage in the ventrolateral funiculus is associated with a higher incidence of at-level allodynia. The Journal of Pain. 11 (9), 864-875 (2010).
- Hubscher, C. H., Johnson, R. D. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. II. Descending pathways. Journal of Neurophysiology. 83 (5), 2508-2518 (2000).
- Hubscher, C. H., Johnson, R. D. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Experimental Neurology. 197 (1), 177-188 (2006).
- Ward, P. J., Hubscher, C. H. Persistent polyuria in a rat spinal contusion model. Journal of Neurotrauma. 29 (15), 2490-2498 (2012).
- Scheff, S. W., Rabchevsky, A. G., Fugaccia, I., Main, J. A., Lumpp, J. E. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. Journal of Neurotrauma. 20 (2), 179-193 (2003).
- Ferrero, S. L., et al. Effects of lateral funiculus sparing, spinal lesion level, and gender on recovery of bladder voiding reflexes and hematuria in rats. Journal of Neurotrauma. 32 (3), 200-208 (2015).
- Smith, R. R., et al. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. Journal of Neurotrauma. 26 (7), 1017-1027 (2009).
- Oelke, M., et al. A practical approach to the management of nocturia. International Journal of Clinical Practice. 71 (11), e13027 (2017).
- Claydon, V., Steeves, J., Krassioukov, A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord. 44 (6), 341 (2006).
- Antunes-Rodrigues, J., De Castro, M., Elias, L. L., Valenca, M. M., McCANN, S. M. Neuroendocrine control of body fluid metabolism. Physiological Reviews. 84 (1), 169-208 (2004).
- Côté, M. -. P., Azzam, G. A., Lemay, M. A., Zhukareva, V., Houlé, J. D. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. Journal of Neurotrauma. 28 (2), 299-309 (2011).
- Dupont-Versteegden, E. E., et al. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 29 (1), 73-81 (2004).
- De Leon, R., Hodgson, J., Roy, R., Edgerton, V. R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. Journal of Neurophysiology. 80 (1), 83-91 (1998).
- Pearson, K. G. . Progress in brain research. 143, 123-129 (2004).
- Gerasimenko, Y., et al. Feed-forwardness of spinal networks in posture and locomotion. The Neuroscientist. 23 (5), 441-453 (2017).
- Courtine, G., et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nature Neuroscience. 12 (10), 1333 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved