A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Use of a Linear Accelerator for Conducting In Vitro Radiobiology Experiments
In This Article
Summary
Clinical linear accelerators can be used to determine biologic effects of a wide range of dose rates on cancer cells. We discuss how to set up a linear accelerator for cell-based assays and assays for cancer stem-like cells grown as tumorspheres in suspension and cell lines grown as adherent cultures.
Abstract
Radiation therapy remains one of the cornerstones of cancer management. For most cancers, it is the most effective, nonsurgical therapy to debulk tumors. Here, we describe a method to irradiate cancer cells with a linear accelerator. The advancement of linear accelerator technology has improved the precision and efficiency of radiation therapy. The biological effects of a wide range of radiation doses and dose rates continue to be an intense area of investigation. Use of linear accelerators can facilitate these studies using clinically relevant doses and dose rates.
Introduction
Radiotherapy is an effective treatment for many types of cancer1,2,3,4. Extra high dose rate irradiation is relatively new in radiation therapy and is made possible by recent technological advances in linear accelerators5. Clinical advantages of extra high dose rate over standard dose rate irradiation include shortened treatment time and improved patient experience. Linear accelerators also provide a clinical setting for cell culture based radiation biology studies. The biological and therapeutic implications of radiation dose and dose rates have been a focus of interest of radiation oncologists and biologists for decades6,7,8. But, the radiobiology of extra high dose rate irradiation and flash irradiation - an extremely high dose rate of radiation - has yet to be thoroughly investigated.
Gamma ray irradiation is widely used in cell culture based radiation biology9,10,11. Radiation is achieved by gamma-rays emitted from decaying radioactive isotope sources, typically Cesium-137. Use of radioactive sources is highly regulated and often restricted. With source-based irradiation, it is challenging to test a wide range of dose rates, limiting its utility in the analysis of the biologic effects of clinical achievable dose rates12.
There have been several studies that illustrate both dose and dose rate effects12,13,14,15,16,17. In these studies, both gamma-irradiation generated from radioactive isotopes or X-rays generated from linear accelerators were used. A variety of cell lines representing lung cancer, cervical cancer, glioblastoma, and melanoma were used. Radiation effects on cell survival, cell cycle arrest, apoptosis and DNA damage were evaluated as readouts12,13,14,15,16,17. Here, we describe a method to define the biological effects of clinically relevant radiation dose and dose rates by delivering X-ray based radiation using a linear accelerator. These studies should be performed with close collaboration between the biologist, radiation oncologist and medical physicist.
Protocol
1. Cell preparation for suspension cell culture
- Culture glioma stem-like cells in stem cell culture media at approximately 5 x 106 cell/10 cm plates in a cell culture incubator with 5% CO2, 95% relative humidity at 37 °C.
NOTE: The cell culture condition is the same throughout all the procedures. The media used in the protocol are complete media. - Two days before scheduled irradiation, collect glioma stem-like cells from the culture plate with a sterile 5 mL pipette into a 15 mL centrifuge tube in a culture hood.
- Centrifuge the collected cells at 200 x g for 3 min in a countertop centrifuge.
- Discard the supernatant and digest the cell pellet (about 1 x 107 cells) with 1 mL of trypsin-EDTA at room temperature for 5 minutes to make a single cell suspension in trypsin-EDTA. Gently shake the bottom of centrifuge tube every 2 minutes during digestion to make sure the cells are digested thoroughly.
- Add 3 mL of stem cell culture media (see the Table of Materials) to quench trypsin. Centrifuge the collected cells at 200 x g for 3 min in a counter top centrifuge. Discard the supernatant and save the cell pellet.
- Resuspend the cells with 5 mL of cell culture media and count the cells with a hemocytometer.
- Plate 5 x 106 cells in two 10 cm plates containing 10 mL of cell culture media.
- Right before the scheduled irradiation, collect the cells and discard the supernatant after centrifugation as described in step 1.3. Re-suspend the cell pellet with 5 mL of cell culture media. Transfer 1 mL of cell suspension into a 35 mm plate containing 2 mL of cell culture media.
NOTE: The total volume of media is 3 mL in the 35 mm plate to make the liquid 1 cm in height in the plate. - Transfer the plated cells in a secondary container, such as a plastic or foam box, to reduce the risk of contamination and bring the cells in the container to the irradiation facility on the utility cart.
- Irradiate the cells as described in step 4.
2. Cell preparation for attached cell culture
- One day before irradiation, in the cell culture hood, remove the DMEM media from the cell culture plate of the attached cell line, such as HEK-293 cells. The media can be suctioned using a Pasteur pipette connected to a vacuum.
- Wash cells with 5 mL of sterile PBS to the culture dish to rinse off residual media.
- Pipette 1 mL of trypsin-EDTA into the culture dish and gently tilt the culture plate to make sure that the entire dish is covered with trypsin-EDTA.
- Trypsinize cells at room temperature for 5 min. Quench the trypsin-EDTA reaction with 3 mL of DMEM media and collect cells with a 5 mL pipette into 15 mL centrifuge tube in the culture hood.
- Centrifuge the collected cells at 200 x g for 3 min in a counter top centrifuge. Discard the supernatant and save the cell pellet.
- Resuspend the cells with 5 mL of DMEM media and count the cells with a hemocytometer.
- Plate 2 x 105 cells in a 35 mm plate using 3 mL of cell culture media to a height of 1 cm of media.
- After 24 h of cell culture in the incubator at 37 °C, transfer the plated cells in a secondary container (i.e., an insulated foam box) to the irradiation facility on a utility cart.
- Irradiate cells as described in step 4.
3. Cell preparation for immunostaining following irradiation
- Thaw commercial extracellular protein matrix on ice at 4 °C overnight. Pre-chill 200 µL pipette tips and 1.5 mL centrifuge tubes at 4 °C overnight.
- Aliquot extracellular protein matrix with pre-chilled pipette tips and 1.5 mL centrifuge tubes at 200 µL per tube.
- Dilute 200 µL of extracellular protein matrix in pre-chilled 20 mL of cell culture media to make 1% extracellular protein matrix media.
- Place a sterilized coverslip (22 mm x 22 mm) in a 35 mm plate. Place 400 µL of 1% extracellular protein matrix media on the coverslip.
- Place the 35 mm plate into the cell culture incubator at 37 °C for 1 h to allow the extracellular protein matrix to polymerize on the coverslip.
- When using suspension cell culture, make a single cell suspension as described in steps 1.1 to 1.5.
- Plate 5 x 104 cells on an extracellular protein matrix-coated coverslip placed in a 35 mm plate. Return plated cells to the cell culture incubator overnight to ensure that cells are supported by the protein matrix. The total volume of the media in the plate should be 3 mL to make the height of media reach 1 cm in the culture dish.
- Observe the cells under a bright field microscope with 10x magnification objective lens. The cells should spread out on the coverslip instead of floating. Transfer the culture dish with plated cells into a secondary container such as a foam box to the irradiation facility on a utility cart and irradiate the cells as described in step 4.
- When using attached cell cultures (for example, HEK-293 cells), digest cells as described in steps 2.1 to 2.6. Place 5 x 104 cells on a sterile cover slip in a 35 mm plate one day prior to the irradiation to make sure cells are fully attached to the coverslip surface by observing them under a microscope as in step 3.9. Use 3 mL of DMEM media to make the liquid 1 cm in height in the plate.
- After growing cells on coverslips overnight or up to one day, transfer the culture dish with plated cells in a secondary container to the irradiation facility. A utility cart may be used to reduce the risk of spillage. Irradiate cells as described in step 4.
4. Irradiation with a linear accelerator (LINAC)
- Using the LINAC's console software, set the accelerator gantry and collimator to 0°, open the X and Y jaws to a symmetrical 20 x 20 cm2 field size and retract the multi-leaf collimators (MLCs) if present.
NOTE: LINACs may have a flattening filter free (FFF) mode, allowing very high dose rates. As the name suggests, this radiation is not uniform (flat), and the high dose rate is only achieved in the center of the beam. In this case a 7 x 7 cm2 field is used. - Place at least 5 cm of water equivalent material on the treatment couch. Place the cell dish to be irradiated at 400 MU/min (standard dose rate) onto the water equivalent material and center it at the LINAC crosshairs.
- Place the cells to be irradiated at a depth of maximum dose in a 6 MV X-ray beam, around 1.5 cm. Place an additional 1 cm of water equivalent material on top of the dish. Combined with the 1 cm of medium throughout which the cells are suspended, this places the cells at an average depth of 1.5 cm.
- Affix the front pointer to the head of the LINAC. Extend the front pointer until it contacts the surface of the buildup material and note the distance. Adjust the table height until the distance from the source to the buildup surface is 100 cm.
NOTE: The distance can be confirmed with the optical distance indicator. Alternatively, the optical distance indicator may be used in lieu of the front pointer to set the source to buildup surface to 100 cm. - Calculate the appropriate number of monitor units (MU) to deliver the desired dose of radiation to the cells and program the accelerator to deliver at 400 MU/min.
NOTE: For the source to surface distance (SSD) setup on a LINAC calibrated to deliver 1 cGy/MU at the depth of maximum dose, the required number of monitor units is calculated using MU = Dose(cGy) / (1 cGy/MU) / OF(20x20), where OF stands for output factor. This calculation will have to be altered for LINACs using alternate calibration setups. - Leave the treatment vault and verify that all other individuals have exited. Vrify that there are no other cells in the room, or they will receive low doses of radiation. Close the vault door.
- Confirm the field size, MU and MU/min at the console and then enable the beam.
- Repeat steps 4.3-4.8 for the cells to be irradiated at higher or lower dose rates.
- To achieve higher dose rates (e.g., 2100 MU/min or greater) with the accelerator, decrease the SSD in order to increase the effective dose rate to the cells according to the inverse square law: DoseRateEffective = Doserate * (100 cm / SSD_New)2.
- For low dose rates (e.g., 20 MU/min), increase the source to surface distance (SSD_New) to decrease the dose rate. For example, the cell culture dish may be placed on the floor of the treatment room.
- Recalculate the monitor unit setting on the accelerator if this setup is necessary, using MU = Dose(cGy) / (1 cGy/MU) / OF(20x20)/ (100 cm / SSD_New)2. For additional information on MU calculations, refer to reference by McDermott and Orton18.
- Determine dose rate in Gy/minute by DoseRate(Gy/Min) = Dose(Gy)*(MU/min)/MU. e.g., 4 Gy delivered at 400 MU/min requires 380 MU, so 4*400/380 = 4.2 Gy/min. See Table 1.
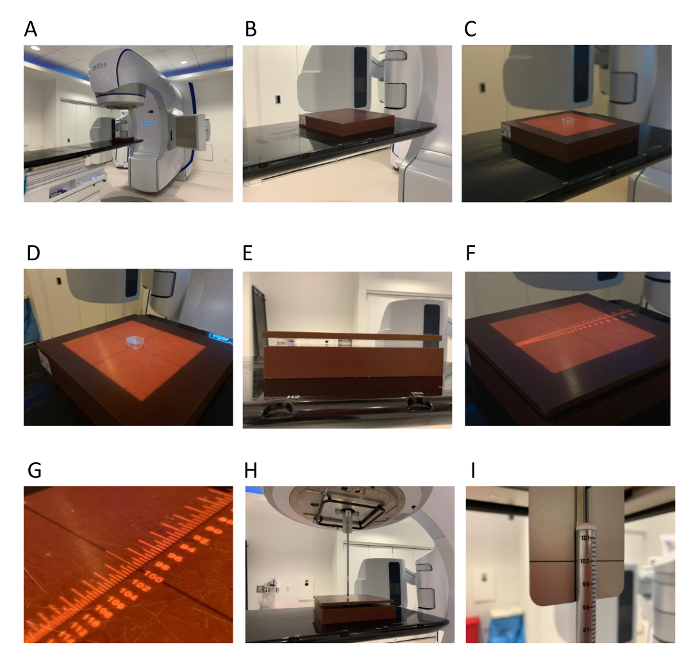
Figure 1: Set-up of the cell culture dish on linear accelerator. (A) A clinical linear accelerator is shown. (B) 5 cm of water equivalent material is placed on the treatment couch. (C) A cell culture dish is placed on the surface of the material. (D) The dish is centered using the accelerator crosshairs in the treatment field shown by the square light field. (E) 1 cm water equivalent material is placed on top of the cell culture dish. The source to surface distance (SSD) is checked using an optical distance indicator (F, G) or a front pointer (H, I). Please click here to view a larger version of this figure.
5. Biological assays after irradiation
- After irradiation, return the cells to the cell culture incubator in the same manner as described above (step 1.9, 2.8 and 3.10).
- As needed, tailor a variety of biological assays to fit into the research project.
NOTE: Here, we show a representative cell cycle analysis16 as an example of a biological assay following irradiation.
Results
To investigate the cell cycle effect of standard dose rate and extra high dose rate irradiation by a linear accelerator, three samples of glioma stem-like cells were prepared using this protocol and collected 24 h after irradiation17: one control sample that was not irradiated (Figure 2A), one sample irradiated with 400 MU/min (monitor unit, 4.2 Gy/min standard dose rate, Figure 2B) to 4 Gy, and another s...
Discussion
Radiotherapy is an integral part of cancer management. Ongoing efforts seek to improve the efficacy and efficiency of radiation treatment. Advancements in linear accelerator technology have provided the opportunity to treat patients with unprecedented accuracy and safety. Because most patients are treated with high energy X-rays from linear accelerators, studies examining the biologic effects of a large range of dose rates performed on linear accelerators may be readily applied to patients. There have been several report...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the Cleveland Clinic Department of Radiation Oncology for use of the linear accelerators. We thank Dr. Jeremy Rich for his generous gift of glioma stem-like cells. This research was supported by the Cleveland Clinic.
Materials
| Name | Company | Catalog Number | Comments |
| Material | |||
| glioma stem-like cell 387 | gift from Dr. Jeremy Rich | ||
| 293 cells | ATCC | CRL-1573 | |
| neuron stem cell culture media | Thermo Fisher Scientific | 21103049 | NeurobasalTM media |
| DMEM | Thermo Fisher Scientific | 10569044 | |
| Fetal Bovine Serum | Thermo Fisher Scientific | 16000044 | |
| Penicillin/Streptomycin | Thermo Fisher Scientific | 15140-122 | |
| Recombinant Human EGF Protein | R&D Systems | 236-EG-01M | |
| Recombinant Human FGF basic | R&D Systems | 4114-TC-01M | |
| B-27™ Supplement | Thermo Fisher Scientific | 17504044 | |
| Sodium Pyruvate | Thermo Fisher Scientific | 11360070 | |
| L-Glutamine | Thermo Fisher Scientific | 25030164 | |
| Tripsin-EDTA | Thermo Fisher | 25200056 | |
| extracellular proten matrix | Corning | 354277 | MatrigelTM |
| Ethanol | Fisher chemical | A4094 | |
| Equipment | |||
| 10 cm cell culture dish | Denville | T1110 | |
| 3.5 cm cell culture dish | USA Scientific Inc. | CC7682-3340 | |
| 22x22mm glass cover slip | electron microscopy sciences | 72210-10 | |
| 15 ml centrifuge tube | Thomas Scientific | 1159M36 | |
| 50 ml centrifuge tube | Thomas Scientific | 1158R10 | |
| 5 ml Pipette | Fisher Scientific | 14-955-233 | |
| pipet aid | Fisher Scientific | 13-681-06 | |
| Vortex mixer | Fisher Scientific | 02-215-414 | |
| Centrifuge | Eppendorf | 5810R | |
| Linear Accelerator | Varian | n/a | |
| water equivalent material | Sun Nuclear corporation | 557 | Solid waterTM |
| Reagent preparation | |||
| DMEM media | 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin G, 100 µg/mL streptomycin in 500 ml DMEM media | ||
| stem cell culture media | 10 ml B27 supplement, 20 µg hFGF, 20 µg hEGF, 2 mM L-glutamine, 100 units/mL penicillin G, 100 µg/mL streptomycin in 500 ml Neurobasal media |
References
- Stupp, R., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 352 (10), 987-996 (2005).
- Stupp, R., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 10 (5), 459-466 (2009).
- Tao, R., et al. Hypoxia imaging in upper gastrointestinal tumors and application to radiation therapy. Journal of Gastrointestinal Oncology. 9 (6), 1044-1053 (2018).
- Gajiwala, S., Torgeson, A., Garrido-Laguna, I., Kinsey, C., Lloyd, S. Combination immunotherapy and radiation therapy strategies for pancreatic cancer-targeting multiple steps in the cancer immunity cycle. Journal of Gastrointestinal Oncology. 9 (6), 1014-1026 (2018).
- Liney, G. P., Whelan, B., Oborn, B., Barton, M., Keall, P. MRI-Linear accelerator raiotherapy systems. Clinical Oncology Journal | The Royal College of Radiologists. 30 (11), 686-691 (2018).
- Hall, E. J. Radiation dose-rate: a factor of importance in radiobiology and radiotherapy. The British Journal of Radiology. 45 (530), 81-97 (1972).
- Steel, G. G., et al. The dose-rate effect in human tumour cells. Radiotherapy & Oncology. 9 (4), 299-310 (1987).
- Ling, C. C., Gerweck, L. E., Zaider, M., Yorke, E. Dose-rate effects in external beam radiotherapy redux. Radiotherapy & Oncology. 95 (3), 261-268 (2010).
- Castro, G., et al. Amotosalen/UVA treatment inactivates T cells more effectively than the recommended gammadose for prevention of transfusion-associated graft-versus-host disease. Transfusion. 58 (6), 1506-1515 (2018).
- Gaddini, L., et al. Exposing primary rat retina cell cultures to γ-rays: An in vitro model for evaluating radiation responses. Experimental Eye Research. 166, 21-28 (2018).
- Simara, P., et al. DNA double-strand breaks in human induced pluripotent stem cell reprogramming and long-term in vitro culturing. Stem Cell Research & Therapy. 8 (1), 73 (2017).
- Wang, Z., et al. A comparison of the biological effects of 125I seeds continuous low-dose-rate radiation and 60Co high-dose-rate gamma radiation on non-small cell lung cancer cells. PLoS One. 10 (8), 0133728 (2015).
- Lasio, G., Guerrero, M., Goetz, W., Lima, F., Baulch, J. E. Effect of varying dose-per-pulse and average dose rate in X-ray beam irradiation on cultured cell survival. Radiation and Environmental Biophysics. 53 (4), 671-676 (2014).
- Karan, T., et al. Radiobiological effects of altering dose rate in filter-free photon beams. Physics in Medicine and Biology. 58 (4), 1075-1082 (2013).
- Sarojini, S., et al. A combination of high dose rate (10X FFF/2400 MU/min/10 MV X-rays) and total low dose (0.5 Gy) induces a higher rate of apoptosis in melanoma cells in vitro and superior preservation of normal melanocytes. Melanoma Research. 25 (5), 376-389 (2015).
- Hao, J., et al. The effects of extra high on glioma stem-like cells. PLoS One. 13 (8), 0202533 (2018).
- Liu, J., et al. Radiation-induced G2/M arrest rarely occurred in glioblastoma stem-like cells. International Journal of Radiation Biology. 94 (4), 394-402 (2018).
- Mcdermott, P., et al. . The Physics and Technology of Radiation Therapy. , (2010).
- Lohse, I., et al. Effect of high dose per pulse flattening filter-free beams on cancer cell survival. Radiotherapy & Oncology. 101 (1), 226-232 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved