Subdural Soft Electrocorticography (ECoG) Array Implantation and Long-Term Cortical Recording in Minipigs
In This Article
Summary
Here, we present a method for the long-term performance and safety assessment of soft subdural electrode arrays in a minipig model, describing surgical method and tools, postoperative magnetic resonance imaging, electrophysiology of the auditory cortex, electrochemical properties of the implant, and postmortem immunochemistry.
Abstract
Neurological impairments and diseases can be diagnosed or treated using electrocorticography (ECoG) arrays. In drug-resistant epilepsy, these help delineate the epileptic region to resect. In long-term applications such as brain-computer interfaces, these epicortical electrodes are used to record the movement intention of the brain, to control the robotic limbs of paralyzed patients. However, current stiff electrode grids do not answer the need for high-resolution brain recordings and long-term biointegration. Recently, conformable electrode arrays have been proposed to achieve long-term implant stability with high performance. However, preclinical studies for these new implant technologies are needed to validate their long-term functionality and safety profile for their translation to human patients. In this context, porcine models are routinely employed in developing medical devices due to their large organ sizes and easy animal handling. However, only a few brain applications are described in the literature, mostly due to surgery limitations and integration of the implant system on a living animal.
Here, we report the method for long-term implantation (6 months) and evaluation of soft ECoG arrays in the minipig model. The study first presents the implant system, consisting of a soft microfabricated electrode array integrated with a magnetic resonance imaging (MRI)-compatible polymeric transdermal port that houses instrumentation connectors for electrophysiology recordings. Then, the study describes the surgical procedure, from subdural implantation to animal recovery. We focus on the auditory cortex as an example target area where evoked potentials are induced by acoustic stimulation. We finally describe a data acquisition sequence that includes MRI of the whole brain, implant electrochemical characterization, intraoperative and freely moving electrophysiology, and immunohistochemistry staining of the extracted brains.
This model can be used to investigate the safety and function of novel design of cortical prostheses; mandatory preclinical study to envision translation to human patients.
Introduction
Neurological impairments and diseases can be diagnosed or treated using electrocorticography (ECoG) arrays. These electrode grids are implanted at the surface of the brain and allow for recording or stimulation of the human cortex1. In the case of drug-resistant epilepsy, for example, they help delineate the epileptic region to resect2. In long-term applications such as brain-computer interfaces, these epicortical electrodes are used to record the movement intention of the brain, to control the robotic limbs of paralyzed patients3. However, current electrode grids are made from stiff metallic blocks embedded in rigid polymeric substrates and do not answer the need for high-resolution brain recordings and long-term subdural biointegration (>30 days). Rather, they create local tissue reactions that lead to fibrotic encapsulation of the implanted device, leading to worse performance over time. Recently, flexible or stretchable electrode arrays using thin polymeric substrates manufactured by microfabrication techniques have been proposed to achieve high performance in long-term implantations by limiting the tissue reaction4,5. However, preclinical studies for these new implant technologies are needed to validate their long-term functionality and safety profile, so that translation to human patients may be envisioned. In this context, minipig and pig models are routinely employed in the development of devices in other medical contexts (e.g., the cardiovascular, skeletal, or gastric systems) due to their large organ sizes and easy animal handling6,7,8. However, only a few applications targeting the brain for neurophysiology are described in the literature, mostly due to surgical approach limitations and integration of the implant system on a living animal9,10,11,12. These are often not compatible with chronic implantation in living animals, as they would require, for example, the development of complex hardware such as implantable embedded electronics. Additionally, they do not investigate the influence of the implant system on the target tissue, which is crucial for the biosafety aspect in translational studies. The porcine model is close to human anatomy in terms of cortical structure, skull bone, and skin thickness13. Furthermore, their ability to learn behavioral tasks makes them a powerful model for investigating functional rehabilitation strategies or sensory perceptions14.
The translation of new technologies and therapies to humans necessitates the assessment of safety and efficacy, as required by competent medical authorities. These are usually described in technical documents and norms15, however they only require the passing of these tests and do not investigate the actual effect of the device implantation or collection of other useful data in parallel to the safety study. For a complete biosafety and performance study on the brain, we present here a longitudinal and systematic collection of brain imaging data, electrophysiological measurements, assessment of electrochemical properties of the implanted electrodes, and postmortem histology in a porcine model. To achieve this, several aspects need to be considered, in order to create a complete experimental model: (i) minimally invasive surgical access for device implantation together with a mechanically stable transdermal port to connect to the electrodes, (ii) a robust electrophysiological recording paradigm that serves as performance output for the implanted electrodes both under anesthesia and in freely moving conditions, (iii) in vivo imaging (computerized tomography [CT] and/or magnetic resonance imaging [MRI]) at different time points to follow the evolution of the brain and implant, as well as the compatibility of the implanted system with the imaging equipment, and (iv) a tissue preparation pipeline to extract the brain for histological analysis.
Here, we report on the method for long-term implantation (6 months) and evaluation of soft ECoG arrays in the minipig model (shown schematically in Figure 1). The soft electrode arrays were presented in our previous reports and are made from thin silicone membranes embedding elastic gold thin films used as electrical tracks16,17. The contact with the tissue is made through a mix of platinum nanoparticles embedded in a silicone matrix for a soft and efficient electrochemical interface to the brain tissue18. The implants are connected through a flexible cable tunneled subdurally through the skull and the skin to a transdermal port that houses the connectors on the head of the animal. The size and shape of the implant can be customized according to the target and the needs of the study. The current electrode strips in this study mirrors the real size of the clinical strips. Clinically available subdural strips and grids were used as comparators using the same approach. The polymeric MRI compatible transdermal port is placed on the skull using a footplate system that anchors it firmly to the skull. Here, we describe in detail the surgical procedure, from subdural implantation of both hemispheres to recovery of the animal. We focus on the auditory cortex as an example target area, where evoked potentials are induced by acoustic stimulation both in anesthetized and freely-moving conditions. At different time points, the animal's brain is imaged in MRI (or CT for the clinical electrodes) under anesthesia and the electrochemical properties of the electrodes are measured. Electrode characterization methods are used to follow the evolution of the implant and the electrode-tissue interface (see Schiavone et al.19 for more details). These include chronoamperometry to probe the stimulation abilities of the electrode contact, electrochemical impedance spectroscopy (EIS) that can indicate the evolution of the resistive and capacitive components of the electrode, and inter-channel resistance measurements to probe for hermetic encapsulation failures. Finally, we have developed a tissue extraction pipeline to perfuse the brain after euthanasia, explant it with the electrodes in place, section it, and perform histological analysis using different inflammation markers. Overall, this method will allow preclinical studies with robust multimodal data collection for future clinical translation of new technologies and therapies on the brain.
Protocol
Surgical and behavioral procedures were approved by the local ethical committee in accordance with the guidelines for the Care and Use of Laboratory Animals and approved by local (Canton of Geneva) and federal (Swiss) veterinary authorities with authorization number GE11120A. Female Göttingen minipigs (n = 7) at 2-6 months of age (5-8 kg) were used in this study.
1. Presurgical planning
- In vitro characterization of the soft implant system
- Chronoamperometry: Record the voltage drop upon the injection of a biphasic current pulse evolution (i.e., the voltage transient [VT]) using an oscilloscope connected in parallel to a pulse generator. Connect the pulse generator sequentially to each electrode and a platinum counter in saline solution (phosphate-buffered saline [PBS] 1x). Refer to step 3.1 for the settings.
- Electrochemical impedance spectrogram: Measure the electrochemical impedance at different frequencies using a potentiometer. Connect the potentiometer sequentially to each electrode using the platinum counter and an Ag/AgCl reference electrode in saline solution (PBS 1x). Refer to step 3.2 for the settings.
- Inter-channel resistance: In the dry state, measure the direct current (DC) resistance between adjacent channels using a handheld multimeter.
- Implant selection: After the three measurements cited above, select the implant along with the following criteria: impedance at 1 kHz below 100 kΩ and no inter-channel resistance below 1 MΩ.
- Sterilization
- Implant sterilization: Place the selected implants individually into sterilization bags along with a sterilization marker and seal them. Employ double packaging to ensure sterility during surgery.
NOTE: In this case, hydrogen peroxide (H2O2) gas sterilization is used due to the short time cycle and low temperature (55 °C). Alternatives are ethylene oxide (ETO) gas or autoclave sterilization, but compatibility with the implant system should be ensured. - Instrument sterilization: Place the cleaned instruments in double sterilization bags or sterile instrument boxes along with sterilization markers. Autoclave sterilization is most common for instruments, but H2O2 or ETO are possible alternatives.
- Implant sterilization: Place the selected implants individually into sterilization bags along with a sterilization marker and seal them. Employ double packaging to ensure sterility during surgery.
2. Surgical implantation of soft ECoG arrays
- Anesthesia
- Premedication: Isolate the animal and fast it overnight. Inject a mix of midazolam at 0.75 mg/kg, atropine at 0.25 µg/kg, and haldol at 0.1 mg/kg intradermally, and wait until the animal is sedated. Weigh the animal before proceeding.
- Installation of intravenous (IV) lead:
- Place the animal on the surgery table on a heating pad. Induce anesthesia by placing a face mask on the animal, using sevoflurane at 3%-3.5%.
- Place electrocardiogram leads on the abdomen, a blood saturation sensor on the tail, and a temperature sensor in the nostril.
- Place an IV lead on an ear vein and confirm blood access using a syringe filled with saline. Ensure the eyes are kept hydrated by using ointment.
- Intubation: Inject a bolus of atracurium at 0.5 mg/kg, ketamine at 1 mg/kg, and fentanyl at 1-2 µg/kg. Place the animal on its back for intubation. Insert a 4.5 mm tube.
- Medication: After intubation, stop the sevoflurane anesthesia and install a propofol infusion at 10 mg/kg/h, fentanyl at 2 µg/kg/h, atracurium at 0.2-0.5 mg/kg/h, and saline at 4-7 mg/kg/h. Start an infusion of mannitol at 1 g/kg/h to reduce brain swelling during the surgery.
NOTE: A multimodal analgesia regimen may be used if recommended by the local animal ethics committee.
- Preoperative x-ray
- Place the animal on its abdomen in the sphinx position. Temporarily remove any metallic object in the vicinity of the brain and skull of the animal (e.g., temperature lead in the nostril).
- Acquire an axial and sagittal plane x-ray with a contrast of the bone. Place a metallic object with known dimensions in the sagittal acquisition field to serve as a scale to measure the skull thickness at the front and back of the brain.
- Identify the location of the frontal sinuses (visible by voids below the skull) and mark the most posterior location on the head of the animal using a permanent marker. This will indicate the furthest point where any craniotomy or screw placement can be done in the surgical approach described below.
- Aseptic field and skin preparation: Shave the entire surface of the head beyond the surgical field. Using a sterile pad, thoroughly scrub the head with betadine. Next, place sterile drapes on the instrumentation table and on the animal to reveal only the surgical window. Finally, scrub the head again with a sterile pad using betadine.
- Craniotomy and durotomy
- Skin cut: Incise the skin with a scalpel knife along the midline. Separate the muscle and periosteum (25 mm laterally from bregma on both sides and 40 mm anterior and posterior to bregma) from the bone using a raspatory and place spreaders to get optimum access for later drilling.
- Measurements and marking: Identify bregma and lambda, and mark them with a sterile surgical pen (Figure 2A, B). Using a sterile ruler, define the bone flap outline centered around the implantation target on both hemispheres. In this specific case, the auditory cortex was chosen, with coordinates -5 mm to -15 mm from bregma and -4 mm to -20 mm laterally. Then, adjust the craniotomy to the size of the implant and anatomical landmarks, limiting the opening size.
- Craniotomy:
- Using a bone drill with a round cutting bit, drill the outline of the craniotomy, taking into account the thickness of the skull measured in step 2.2. Irrigate the drilling location with saline solution to avoid overheating of the bone.
- Carefully drill the outline homogeneously until reaching the dura mater. At the first breakthrough, finish drilling the outline until it has thinned enough to almost break through. Then, use a flat spatula (either on the midline side or the lateral side) to break away the bone flap in one piece, using the craniotomy edge as leverage. If too much resistance is encountered, continue thinning the bone.
- Place the bone piece in sterile saline.
- Once the bone flap is removed, carefully chip the edge of the craniotomy away, using a Kerison to avoid the sharp bone edge from cutting into the dura mater.
- If excessive bleeding is encountered on the dura mater or the bone, use Gelfoam or bone wax, respectively. Place a wet compress (standard pad in sterile saline solution) in the craniotomy and repeat this step on the other hemisphere (Figure 2B).
- Durotomy:
- Using the needle from a 6-0 suture kit, carefully pierce and lift the dura mater at the anterior or posterior end of the craniotomy halfway between the medial and lateral side and create the beginning of an incision with the stab knife.
- Then, using a small flat spatula inserted in the subdural space acting as a cutting base to protect the cortex, create an anteroposterior slit in the dura mater by advancing simultaneously with both tools. Ensure that the slit is slightly larger than the width of the implant (Figure 2C). If any bleeding or damage occurs at this step, cover it with gel foam and wait until it stops.
NOTE: The slit trajectory should be adapted if large blood vessels are present in the dura mater to avoid bleeding.
- Implantation
- Device insertion:
- Irrigate the implant (Supplementary Figure 1A) with saline on both sides so it slides into the subdural space more easily. Place the implant above the dura mater slit and, with small forceps, subdurally insert the device by sliding it sequentially on each edge.
- Carefully hold the pedestal end of the device and advance with the implant in order not to create tension hindering the insertion. When the connector edge is located on top of the slit, stop the insertion.
- Secure the implant: To secure the implant in place, place a titanium bridge over the cable after the edge of the craniotomy or in the anchoring wings and secure it with one or two titanium screws using the appropriate screwdriver (Figure 2D).
- Ground placement: Carefully remove 1 cm of the insulation of the ground wires and insert it epidurally at the posterior end of the craniotomy (or any epidural location far from the cortex of interest or large blood vessels) (wire in Figure 2E)
- Repeat steps 2.4.4. and 2.5.1.-2.5.3 on the contra-lateral hemisphere.
- Intraoperative x-ray to confirm placement:
- Place a wet pad (standard pad in sterile saline solution) over the surgery location to keep the tissue hydrated. Next, place a sterile surgery drape to cover the head of the animal.
- Take plane x-ray images (axial and sagittal) to ensure the implants are well-placed and are not folded, using the x-ray markers as indicators. If not, remove the drape and explant the device to insert it again (follow steps 2.4.4. and 2.5.1.-2.5.3 again).
- Dura mater closure: Suture the dura mater carefully around the implant cable using a 3-0 resorbable suture and a small needle holder. Bring the two dura mater edges together as much as possible without tearing through the thin membrane with the suture wire (Figure 2D, E).
- Bone flap placement: Fix a titanium bridge on the anterior and posterior part of each bone flap using a titanium screw. Be careful to plan the positioning of the Ti-bridges with respect to the placement of the footplate legs in the next steps. Screw the end of the titanium bridges to the skull (Figure 2F, G).
- Device insertion:
- Pedestal and footplate placement
- Positioning: In this configuration, the footplate has six legs with two screw holes each (Supplementary Figure 1B). Plan the placement of the footplate on the skull to optimize the location of the screws (avoid placing them at the edge of the craniotomy or in the temporal muscle). Skip the holes in the legs if they cannot be screwed in.
- Footplate securing: Screw in the titanium screws of the footplate until it is firmly in place (see Figure 2H).
- Pedestal placement: Remove the titanium bridges over the connection cables and flip over the pedestal to land on the footplate. Screw the pedestal onto the footplate. Check that the pedestal is firmly in place (Figure 2I).
- Suture and closure
- Cleaning of the wound: Clean the subcutaneous space of any bone or other debris by flushing with saline. Cut away some skin around the pedestal edges to create a round edge following the cylinder.
- Subcutaneous sutures: Remove the spreaders and fold the skin flaps together. Create subcutaneous sutures with a 4-0 non-resorbable suture wire, 3 mm apart using simple interrupted sutures or simple continuous sutures. Start away from the pedestal, moving toward it on both sides of the incision.
- Dermal sutures: Suture the skin using a 6-0 non-resorbable suture wire, with sutures 5 mm apart. Start away from the pedestal, moving toward it on both sides of the incision. Take care to achieve good tissue apposition between the two skin flaps and near the pedestal edge to avoid a void (Figure 2J).
- Wound dressing: Clean the wound area again with a sterile pad and betadine. Apply a self-adhesive sterile bandage over the wound.
- In vivo measurements: For in vivo measurements, follow sections 3, 4, and 5.
- Awakening: After all the measurements have been performed, take the animal off all anesthetics but keep under ventilation. For analgesia, apply a buprenorphine patch (25 mg/h) for 24 h. Place the animal on a heating pad covered with drapes to speed up the wake-up time. When spontaneous breathing is recovered, extubate the animal and put under an oxygen face mask until consciousness is recovered (which can take 1 to 4 h).
- Postoperative animal care: For 5 days, keep the animal under close surveillance. Give a dose of cephalexin at 75 mg twice daily with food, separated from other animals. Carry out disinfection of the wound daily by applying copious amounts of betadine with soaked sterile pads (best done during feeding).
NOTE: Long-term care and housing: The operated animal is kept isolated for 24 h. It is put back in its original social group if the animal is well enough to interact socially with its peers. Daily observation of the pedestal and skin opening needs to be conducted to follow the integration of the device on the head. When appropriate, clean the location around the pedestal with copious amounts of betadine.
3. In vivo characterization of the soft implant
- Chronoamperometry: Record the voltage drop upon the injection of a biphasic current pulse evolution (i.e., the VT) using an oscilloscope connected in parallel to a pulse generator. Connect the pulse generator sequentially to each electrode and the ground wire. Perform the stimulation pulse at 100 µA with a pulse width of 300 µs at 100 Hz.
- Electrochemical impedance spectroscopy: Measure the electrochemical impedance at different frequencies using a potentiometer. Connect the pulse generator sequentially to each electrode, using the ground wire as both the counter electrode and the ground. Set the excitation voltage to 200 mV and the frequency range from 1 Hz to 1 MHz, with three points per decade.
- Inter-channel short measurements: Measure the DC resistance between adjacent channels using a handheld multimeter. In vivo, the inter-channel DC resistance is only measured to verify that no shortages below 1 kΩ appear, indicating total encapsulation failure.
4. Electrophysiological recording
- Spontaneous activity: Plug the wireless recording system through the pedestal and record the baseline activity for 2-3 min. These recordings will serve as a control to analyze the auditory evoked potentials.
- Auditory evoked potentials: In addition to the wireless system, insert speakers in a closed field in the animal ears. Play tone burst acoustic stimulation at different frequencies (ranging from 200-20,000 Hz) at around 70 dB sound pressure level (SPL) over 120 repetitions. Then, average the recordings and align them over the stimulus period for analysis.
- Sensory evoked potentials: Place the needles in the snout at three different positions. Evoke sensory potentials by stimulating the snout for ~30 s with the pulse generator at different amplitudes to obtain the recruitment curves.
5. In vivo imaging
- Animal transport: Keep the animal under propofol anesthesia, as described in step 2.1. Use a transport cart that houses a ventilator, syringe pump, and vital signs monitor to transport the animal from the surgery room to the imaging facilities and back.
- Computed tomography x-ray scan: Place the animal on the scanner table and remove any metallic object around the head (e.g., the temperature sensor). Acquire a CT scan at the smallest resolution (0.4 mm slice thickness) using isometric acquisition with automatic current and voltage selection for bone contrast.
- Magnetic resonance imaging: Remove all equipment containing metal from the animal (use MRI-compatible IV leads and intubation tube). Keep the animal ventilated and anesthetized under sevoflurane at 3%-3.5%, using a ventilator situated outside the MRI chamber and linked through a long tube to the animal. Before the first sequence, inject a bolus of fentanyl at 1-2 µg/kg. Use three isometric sequences at the smallest resolution: T1-, T2- and turbo spin echo (TSE)-weighted sequences (parameters shown in Supplementary File 1).
6. Freely moving recording
- Follow the same procedure described in section 4 for recording awake signals from the brain. Plug the wireless headstage by either holding the animal in the experimenter's arms or feeding the animal with treats to distract it. Provide the acoustic stimulation using external speakers placed close to the animal.
7. Perfusion and tissue preparation
- OPTIONAL: If the animal is not already under anesthesia, follow step 2.1 for the anesthesia protocol.
- Insertion of a catheter in the carotid artery for perfusion (Supplementary Figure 2)
- Carotid artery and jugular vein dissection: Cut the throat at the midline using a cauterizer/cutter. Cut the skin first and then the muscle following the middle white line (no vessels underneath) (Supplementary Figure 2A).
- Open further, using fingers to make space around the trachea and under the muscle. Look for the carotid artery (beating and pinkish); the vagal nerve is sometimes also around (white), and the jugular vein can be underneath or on the side (red). Place the spreaders (see Supplementary Figure 2B).
- Start the dissection of the carotid artery using fine forceps and round scissors. Use scissors to open conjunctive tissue. If there are no blood vessels, cut; if there are blood vessels, cauterize and move forward (Supplementary Figure 2C, D). When dissected enough, use a clamp to go underneath the carotid artery so it is completely isolated (Supplementary Figure 2E).
- Repeat the same operation with the jugular vein (Supplementary Figure 2F).
- When both vessels are fully dissected and isolated, place suture wire around them. Do not close them yet. Two sutures around the carotid, one at the very base (suture 1-heart side for brain perfusion) and one on the other side (suture 2), are shown in Supplementary Figure 2G.
- Place one suture around the jugular vein (suture 3), not closed; mark the wires with tape for sectioning of the vein later.
- Close suture 1 very firmly, otherwise it will bleed (Supplementary Figure 2H). Tie three knots. Place a clamp on the wire to put weight and create tension in the carotid artery.
- Clamp the carotid artery using a vessel clamp on the opposite side of the carotid artery dissection (brain side for brain perfusion; Supplementary Figure 2I). Suture 2 is in the middle, not closed yet.
- Use black forceps to catch and pull the carotid artery. Using fine scissors, section half of the carotid artery near the base of the dissection (near suture 1, heart side; see Supplementary Figure 2J). Ensure that the section is as neat as possible and reaches the vessel itself, not only the "sheath"; otherwise, the catheter will not go through. Insert the catheter as shown in Supplementary Figure 2K.
- Flush and fill the catheter, first with PBS, so there is no air left in the catheter (Supplementary Figure 2K inset).
- Close suture 2 firmly enough so it does not go away, but not too much so that the catheter can still move a bit (Supplementary Figure 2L). Then, remove the vessel clamp, finish inserting the catheter as far as possible, and finalize the closure of suture 2 (close firmly).
- If desired, use suture wires at suture 1 to attach the base of the catheter to the skin at the exit of the wound as an extra security step. Then, transfer the animal to the perfusion zone and plug it to PBS/heparin. When the perfusion starts, pull suture 3 and cut the jugular vein
- Euthanasia: Deliver pentobarbital (90 mg/kg) intravenously and flush the line with saline to ensure the full dose is administered successfully.
- Perfusion: Using a perfusion pump (200 mL/min) plug the carotid catheter into PBS/heparin (1 L for a 15 kg pig) and then into PBS containing 4% paraformaldehyde (PFA) (5 L for a 15 kg pig). When the perfusion starts, pull suture 3 and cut the jugular vein.
- Tissue collection
- Beheading: When perfusion is over, detach the animal's head from the body using a scalpel, by cutting through the skin and muscle and inserting the blade between the first and second vertebrae.
- Postfixation: Plunge the head in 4% PFA in PBS for another 48 h at 4 °C and then transfer to PBS before brain extraction.
- Brain and implant extraction:
- Remove the skin using a scalpel and start to cut the bone carefully using a rongeur starting from the first vertebrae, following the spinal cord to the cerebellum. Once the cerebellum is exposed, carefully remove the temporal bones and expose the parietal and frontal lobes.
- At this point, screw the pedestal off the footplate and cut the feet of the footplate with pliers. Remove the bone near the cable entry into the skull to free the implant system from the bone, without pulling the implants out of the dura mater exit.
- If the implant cables are embedded into the bone, cut the cable as close as possible to the exit. Once enough brain surface is exposed, carefully cut the dura following the midline using small scissors.
- Free the implant cables from the dura mater exit. Take photographs from the implant placement on the brain. Then, use a small spoon to detach the brain from the cranial nerves below. Carefully extract the brain. Remove the implants from the brain.
- Postfixation of the brain: Depending on the perfusion quality, postfix the extracted brain again for 24 h in 4% PFA in PBS at 4 °C. Keep in 0.1 M PBS at 4 °C until the brain is cut.
- Brain cut: Separate the two hemispheres using a razor blade. Then, cut the brain orthogonally in four different pieces. Cut the implanted zone in half to obtain two blocks containing the implanted zone and a control area. Store the other two blocks if more control slides are needed.
- Brain cryoprotection and freezing: Transfer the brain blocks to a solution of 15% of sucrose first and then 30% of sucrose at 4 °C until the brain plunges and reaches equilibrium. Then, freeze the tissue in isopentane at -55 °C in a tissue freezing system.
8. Histology
- Tissue sectioning
- Cryostat: Place the frozen brain in a cryostat and trim until full sections are achieved. Then, slice the brain into 40 µm sections and immerse in groups of three in 0.1 M PBS in well plates. Carefully note the order of the plates.
- Section selection: Select the sections depending on the zone to analyze (implanted region or control zone). Take the sections sequentially out of the well plates, using a fine brush to inspect them for damage. Place them in new well plates filled with 0.1 M PBS for further staining.
- Immunohistochemistry
- Preparation: Incubate the sections in 0.3% Triton X/PBS for 15 min, followed by 3% bovine serum albumin (BSA)/PBS for 1 h at room temperature (RT).
- Primary antibodies: Incubate the tissues with primary antibodies for 48 h at RT (anti-GFAP, rat, diluted at 1/300; anti Iba1, rabbit, diluted at 1/400; anti-NeuN, guineapig, diluted at 1/1,000; all in 1% BSA/PBS). Cover the well plates with aluminum foil.
- Washing: Wash the wells with 0.1 M PBS three times for 5 min.
- Secondary antibodies: Incubate the tissues with secondary antibodies for 2 h at RT (Alexa Fluor 488, Alexa Fluor 647, Alexa Fluor 555; all diluted at 1/400 in 1% BSA/PBS).
- DAPI (4′,6-diamidino-2-phenylindole): Incubate the tissues with DAPI for 15 min (1/1,000 in 1% BSA/PBS).
- Washing: Wash the wells with 0.1 M PBS five times for 15 min.
- Mounting: Mount the slides using mounting media and a coverslip. Keep the slides in the dark in a fridge at 4 °C.
- Imaging
- Whole slide imaging: Image the slides at 10x magnification (objective working distance value = 3,100 µm) using a slide scanner microscope at three different wavelengths (640 nm, 560 nm, 485 nm). All power and gain information can be found in Supplementary File 2.
- Microscopic imaging: Image the region of interest at 20x magnification (apochromat 20x/0.8 M27) using a confocal microscope at four wavelengths (Alexa Fluor 647, DAPI, Cy3, EGFP). All power and gain information can be found in Supplementary File 3.
Representative Results
In order to confirm the placement (Figure 3A) and functionality of the devices, electrophysiological recordings are performed intraoperatively after pedestal placement. The baseline signal is first acquired over 2 min with no stimuli as the control of basal activity. Secondly, the animal is acoustically stimulated with a tone burst at different frequencies (500-20,000 Hz), and the raw data is averaged over the stimulus period to map auditory evoked potentials across the array (e.g., at 800 Hz compared to baseline; Figure 3B). The data shown here are unprocessed, but if too much noise is present, notch and bandpass filters can be applied. Typical sources of noise in the surgical theater include heating pads, plugged drills, and suction or cauterizers (among others) that should be removed prior to acquisition. In awake recordings, large muscle movement around the head, such as chewing, should be avoided for cleaner data sets.
This protocol was applied at every recording time point, and signals for a single channel could be compared over time. One example is illustrated in Figure 3C, showing the robustness and evolution of the response. The recording capacity of each contact over the time course of the experiment can be evaluated by calculating the standard deviation of the baseline signal at every time point (Figure 3D). In this study, the signal-to-noise ratio decreased and settled between day 0 and month 6, despite some variability due to the limited duration of the recording period (i.e., 2 min). This can be further correlated to electrode impedances.
The in vivo imaging is performed postoperatively to assess the brain state and implant positioning. In the first iteration of the protocol, no intraoperative x-ray was performed, resulting in a folded device, as is visible in Figure 4A on a T1-weighted MRI sequence (see in addition Figure 4B). No behavioral change was observed in the animal, but over time, this resulted in a fibrotic encapsulation around the device due to the macroscopic compression of the brain around the implant location (Figure 4C). After this experience, intraoperative x-ray was introduced, as shown in Figure 4D, where the radiopaque markers (black bars visible on the implant in inset Figure 4D) are shown to be well positioned. The surface of the brain is then intact, as can be observed in the postoperative MRI in Figure 4E. Overall, with this implant and pedestal system, whole-brain imaging is possible. Different sequences in the coronal planes enable to see anatomical structures (Figure 4F,G; T1 and T2 MRI sequences) or the presence of liquid and blood around the implant (Figure 4H; TSE-weighted MRI sequence). The pedestal system creates almost no artifacts, except for some small black-contrasted voids around the titanium screws (see Figure 4G). Additionally, clinical electrodes are used as comparators in this study, but cannot be imaged in the MRI due to heating and safety concerns. Therefore, CT scans are acquired on these animals, as shown in Figure 4I. The electrodes are clearly visible, and the pedestal system is not influencing the image quality.
After the implantation period, the animal is perfused, and the brain extracted. In this study, the analysis of the inflammatory response is performed on each hemisphere independently. Cutting the brain in half is easier for tissue preparation before sectioning, and has the advantage that sections can be mounted on standard microscopy slides. One example of a brain sample is shown before (Figure 5A) and after (Figure 5B) cutting in blocks. The outline of the implant is clearly visible and has created a small dent in the brain. By cutting in parallel planes, the tissue is then already aligned to the cryostat, and sections can readily be cut without tissue loss for trimming (Figure 5C). After staining, the whole tissue section is imaged (Figure 5D), where for example, the neuron layer is clearly visible in detail (see NeuN marker). Whole sections are fragile and can sometimes lead to some loss of tissue (see the bottom of Figure 5D), but the area of interest is intact. On a closer view, enabled by confocal microscopy imaging at 40x, the cells are clearly defined and enable fine investigation of inflammatory markers, for example (Figure 5E). Further quantifying analysis can be performed to compare inflammation between control and implanted hemispheres. Figure 6 shows the electrochemical characterization of the implanted electrodes. The In vitro electrochemical impedance spectroscopy of the soft electrode array with impedance modulus and phase is shown in Figure 6A and the impedance modulus at 1 kHz over 6 months of implantation is shown in Figure 6B.
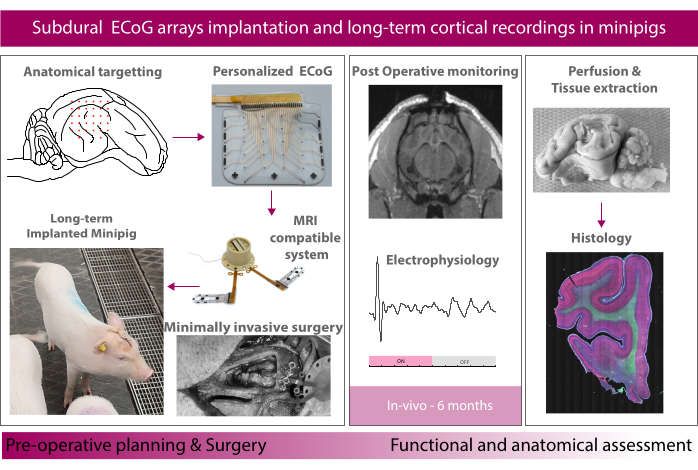
Figure 1: Schematic of the experiment. Please click here to view a larger version of this figure.
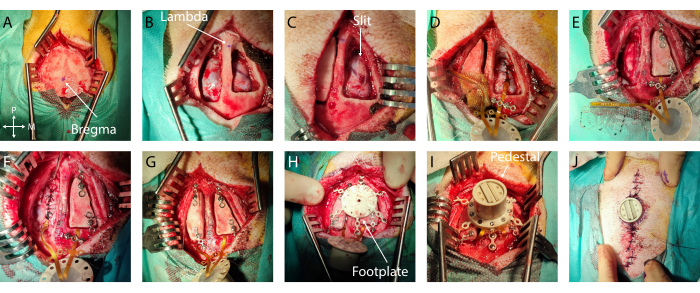
Figure 2: Minimally invasive implantation of soft ECoG onto the brain. (A) Surgical access to the skull, with indication of bregma. (B) Bilateral craniotomy with visible dura mater. (C) Slit durotomy on the first hemisphere. (D) Subdural implantation of soft ECoG and dura mater closure. (E) Slit durotomy on the second hemisphere. Bone flap fixation on the first hemisphere using titanium bridges. (F) Implantation of soft ECoG on the second hemisphere and dura mater closure. (G) Bone flap fixation on the second hemisphere. (H) Footplate positioning on the skull. (I) Pedestal fixation onto the footplate. (J) Skin closure around the pedestal base. Please click here to view a larger version of this figure.
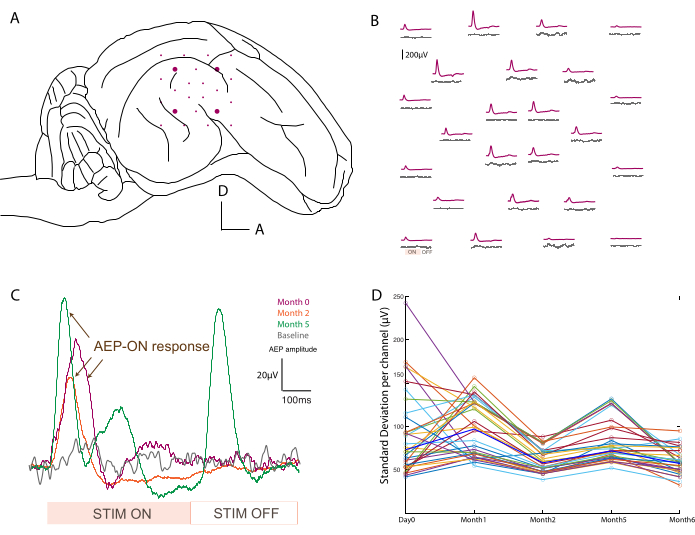
Figure 3: Recording of auditory evoked potentials. (A) Schematic of electrode placement at the surface of the temporal lobe. (B) Representative mapping of baseline activity (gray traces) and auditory evoked potentials in response to an 800 Hz tone burst stimulation (purple trace). Each average corresponds to one channel on the soft ECoG array. The averaging is triggered on the analog input signal from the sound stimulation. "ON" and "OFF" acoustic stimulation periods are noted on one channel in the bottom left. (C) Evolution over time (day 0, month 2, and month 5) of a single channel response after acoustic stimulus, compared to baseline signal when no stimulus is presented (gray). The averaging is triggered on the analog input signal from the sound stimulation. The "ON" and "OFF" stimulation periods are noted at the bottom. The evoked potential of the "ON" stimulation is marked with arrows. (D) Standard deviation per channel (colored dots) per time point of the baseline recording. Median values are represented in bold blue. Please click here to view a larger version of this figure.
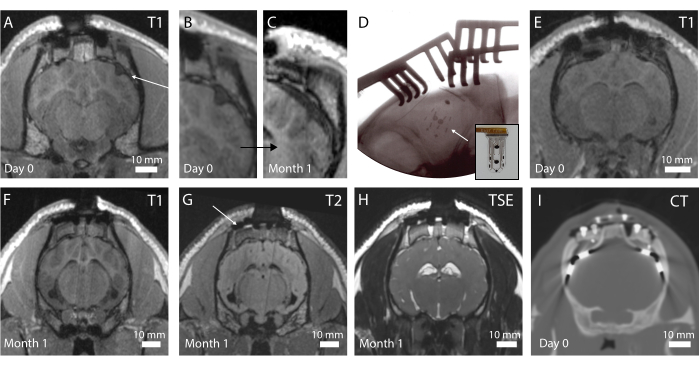
Figure 4: In vivo imaging of the brain and implanted electrodes. (A) Postoperative T1-weighted MRI in the coronal plane. An arrow indicates a folded implant. (B) Magnified portion of A, where the folding of the implant creates a dent in the brain. (C) T1-weighted MRI at 1 month implantation, showing compression of the brain due to the fibrotic encapsulation of the brain at the same location as C. (D) Intraoperative plane x-ray verifying implant placement and no folding, as observed by the radiopaque marker placement. Inset: Photograph of implant with radiopaque marker visible. (E) Postoperative T1-weighted MRI in the coronal plane with optimal implant placement. (F) T1-weighted MRI at 1 month implantation. (G) T2-weighted MRI at 1 month implantation. An arrow shows the imaging artifact from the titanium screws holding the footplate in place on the skull. (H) TSE-weighted MRI at 1 month implantation. (I) CT scan of the animal implanted with the clinical electrodes. Please click here to view a larger version of this figure.
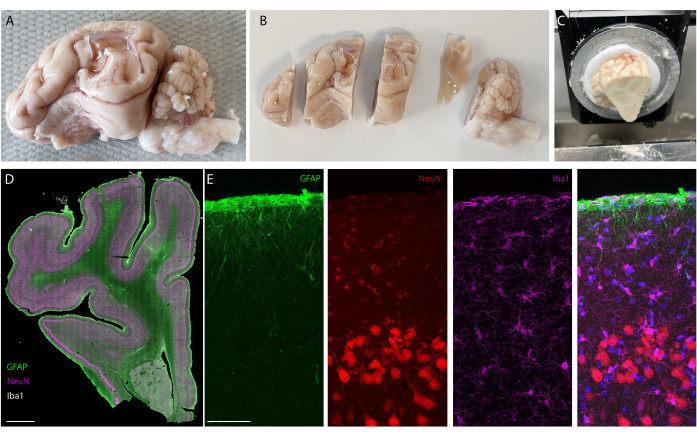
Figure 5: Histology analysis of the brain after long-term implantation. (A) Photograph of an explanted and perfused brain-left hemisphere. (B) Perfused brain cut in blocks prior to the freezing step. (C) Picture of whole block sectioning setup on the cryostat; the entire "pre-cut blocks" can be sectioned. (D) Immunostaining imaging of the whole hemisphere (slide scanner, 20x objective) and(E) zooming on the first layers of the cortex (confocal imaging, 40x objective) showing glial cells, astrocytes, and neurons. Please click here to view a larger version of this figure.
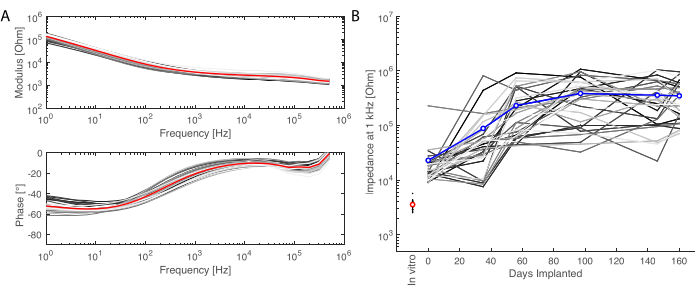
Figure 6: Electrochemical characterization of the implanted electrodes. (A) In vitro electrochemical impedance spectroscopy of the soft electrode array (small grey lines for each channel, the average in red) with impedance modulus (top) and phase (bottom). (B) Evolution of the impedance modulus at 1 kHz over 6 months of implantation (mean in blue; grey lines are the individual channels; the in vitro measurement is given as reference in red). Please click here to view a larger version of this figure.
Supplementary Figure 1: MRI-compatible pedestal. (A) Chronic MRI-compatible transdermal connection system (pedestal) to access the soft electrode array. (B) Pedestal with electrodes mounted on the footplate for skull anchoring. Inset: Details of the footplate. Please click here to download this File.
Supplementary Figure 2: Surgical access for optimal perfusion of the brain. (A) Skin cut and access to the location of the carotid artery and jugular vein. (B) Dissection of the tissue around the blood vessels. (C,D) Identification and dissection of the tissue around the carotid artery and jugular vein. (E) Isolation of the carotid artery from the tissue beneath. (F) Isolation of the jugular vein from the tissue beneath. (G) Suture wire placement around the carotid artery (suture 1 and suture 2) and the jugular vein (suture 3). (H) Closure of suture 3 at the base of the carotid artery (heart side) to avoid bleeding during opening of the vessel. (I) Clamping of the carotid artery at the opposite side from H. (J) Sectioning of the carotid artery. (K) Inserted catheter in the opening from J. Inset: Primed catheter with saline flushed from a syringe to the catheter tip. (L) Closure of suture 2 to maintain the catheter in place and along the artery. Please click here to download this File.
Supplementary File 1: Parameters for T1- (pages 1-2), T2- (pages (3-4) and TSE-weighted (pages 5-6) MRI sequences, respectively. Please click here to download this File.
Supplementary File 2: Metadata for slide scanner for whole slide imaging of stained brain slices. Please click here to download this File.
Supplementary File 3: Metadata for confocal imaging of magnified section of stained brain slices. Please click here to download this File.
Discussion
We report here a method for long-term implantation and evaluation of soft ECoG arrays. In this study, we have designed a consistent, minimally invasive surgical approach for bilateral implantation of functional electrode grids over the temporal lobes (here, targeting the auditory cortex). We first evaluated the functionality of the grid by successfully recording evoked potentials over the time course of the study (6 months) and tracking the electrochemical properties of the electrodes (see Figure 6). Secondly, we assessed the biosafety of the grids, in vivo by using MRI and establishing a fully MRI-compatible system, and postmortem by designing a protocol for tissue collection and immunostaining.
To minimize invasiveness, we optimized the size of the craniotomy window. In order to reach the auditory cortex located on the temporal lobe and to avoid resecting the temporal muscle, we have developed a technique to slide the implant under the dura. This technique allows to drastically reduce the surface of the exposed brain and still reach far-away targets. While this type of implantation may seem blind, the implementation of radiopaque markers on the devices that are visualized in the intraoperative plane x-ray allows for verification of positioning, and ensures the array is not folded under the dura mater. The subdural sliding has proven to be safe in most of the repetitions we have performed. Additionally, the durotomy in a slit approach minimizes brain bulging during the time the craniotomy is open and facilitates the closure around the implant without requiring additional material such as artificial dura mater, which could bias the inflammatory response. Finally, the strength of this surgical approach is its ability to be transposed to different cortical regions. Playing with coordinates, the craniotomy position, and the device size, which can all be adjusted, enables this method to target most of the cortex area.
The surgical method presented here, along with functional assessment and investigation of the biointegration over time, is not limited to the soft electrode technology used in this report. Other subdural electrodes that are being developed for human translation could be evaluated with the same protocol. The strength of this method relies on the fact that most of the pieces, such as the cable and pedestal, are modular, personalizable, and can be adapted to the specific device under test. Additionally, intracortical or deep penetrating probes could also be used instead of or in combination with the subdural electrodes, as this only requires adjusting the craniotomy and durotomy geometry. The long-term results can then be compared to their clinical counterparts, as we have done here.
One of the major limitations of the presented method is the presence of skull sinuses in minipigs, which develop over the course of the first year12. In that regard, important aspects to take into account include the age of implantation and also the size of the animal. Performing craniotomies in the adult skull breaks sinuses' integrity and leads to a high risk of major infection in chronic settings. Such sinuses are visible in the plane x-ray and CT scan preoperatively. On the other hand, performing chronic implantation too early, in an animal that is too small, is also not optimal when the skull is undergoing massive growth and remodeling. We hypothesized that these "skull movements" post-surgery could cause the implant to move and fold, which ultimately is detrimental to the experiment. We have found here that Göttingen minipigs, approximately 5-6 months old (and 8 kg) at the time of implantation, should give the best results.
For evaluating the performance of the implanted ECoG for electrophysiological recordings, we have set up a rapid protocol for auditory evoked potential (AEP) recording that can be used in freely moving animals and under sedation. It consists of presenting a series of acoustic tone bursts at specific frequencies over the course of a few minutes. The advantage of such a protocol is the fact that it can be tuned to the available length of recording by reducing the number of frequencies probed. One challenge when recording cortical signals under anesthesia is that the level of consciousness of the animal should be taken into account when analyzing and comparing the data.
The protocol for perfusion was adjusted over time by observation of the extracted brain's quality. Indeed, we found it easier to catheterize the carotid artery only, and not the jugular vein. Initially, the literature presents methods where the jugular vein is catheterized to drain waste20. Practically, this limits the flow out of the brain and leads to poorer extraction of blood and the overall quality of perfusion. By cutting the jugular vein and leaving the liquid to escape in a large container where the animal lies, the efficiency of the perfusion increases.
We have developed a robust tissue preparation method that works with antibodies routinely used for inflammation tracing. We have separated the two hemispheres for practical reasons, as half the pig's brain fits on standard microscope slides and is thus compatible with most imaging equipment available in histology laboratories. By cutting the brain in blocks, direct access to the zone of interest is made possible without requiring further cutting of the whole brain or trimming extensive parts of the tissue. The brain slices at 40 µm can be pooled in standard well plates and stained in a free-floating fashion without major protocol changes from other species' immunostainings. Full brain immunostaining could also be envisioned by using, for example, CLARITY methods21.
Overall, this protocol, which covers personalized implant design to implantation, functionality follow-up, and biosafety assessment, is robust and consistent. We demonstrated here its feasibility to study the auditory system, but it can be transposed to test other physiological functions. Moreover, the strength of our method resides in the fact that it is not restricted to minipigs, but fully transposable to other species such as sheep, goats, or non-human primates. To a certain extent, it can also be easily adapted to rats.
Disclosures
F.F. and S.P.L. are co-founders and shareholders of Neurosoft Bioelectronics SA developing soft electrode arrays.
Acknowledgements
The authors would like to acknowledge financial support from the Bertarelli Foundation and the SNSF Sinergia grant CRSII5_183519. The authors would also like to thank Katia Galan of EPFL for her help on developing the staining protocol for the histology, the staff at the Neural Microsystems Platform of the Wyss Center for Bio and Neuroengineering in Geneva for their help with the fabrication processes, the staff of the of animal platform in the University Medical Center (CMU) at the University of Geneva (UNIGE) for animal care, surgical assistance, and postoperative management of the minipig (John Diaper, Xavier Belin, Fabienne Fontao, and Walid Habre), the team members of the Center for Biomedical Imaging (CIBM) at the University of Geneva (Julien Songeon, François Lazeyras, and Rares Salomir), the staff members of the Pathology Department at the University Hospital Geneva (HUG) (Sami Schranz, Francesca Versili, Ruben Soto, and Coraline Egger), and Blaise Yvert from Université Grenobles-Alpes for his input and exchanges on chronic minipig experiments. The authors would like to acknowledge the help of employees of Neurosoft Bioelectronics SA, for their help with the fabrication process and for their help in the minipig experiments (Benoit Huguet and Margaux Roulet).
Materials
| Name | Company | Catalog Number | Comments |
| Bone drill | BBraun | Elan 4 with GA861 handpiece | |
| Bone drill bit | BBraun | Neurocutter GP204R | |
| Bonewax | Ethicon | W31G | |
| Catheter | Venisystems | Abbocath 14G | |
| Confocal Microscope | Zeiss | LSM 880 | |
| Cryostat | Leica | CM1950 | |
| Gelfoam | Pfizer | Gelfoam | |
| Insert speakers | Etymotic | Etymotic ER2 insert Earphones | |
| Multimeter | Fluke | Fluke 1700 | |
| Oscilloscope | Tektronix | MDO3014 Mixed Domain Oscilloscope | |
| Perfusion pump | Shenzen | LabS3/UD15 | |
| Potentiostat | Gamry Instruments | Reference 600 | |
| Primary Antibody Anti-GFAP | Thermofischer | Anti-GFAP, Rat, # 13-0300 | |
| Primary Antibody Anti-Iba1 | Fujifilm | Anti Iba1, Rabbit, 019-19741 | |
| Primary Antibody Anti-NeuN | SigmaAldrich | Anti-NeuN, GuineaPig, ABN90 | |
| Pulse Generator | AM Systems | Model 2100 Isolated Pulse Stimulator | |
| Recording headstage | Multichannel systems | W2100-HS32 | |
| Recording system | Multichannel systems | W2100 | |
| Screwdriver | Medtronic | Handle: 001201, Shaft: 8001205 | |
| Secondary Antibody 488 | Thermofischer | Goat anti-Rat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488, # A-11006 | |
| Secondary Antibody 555 | Thermofischer | Goat anti-Guinea Pig IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 555, # A-21435 | |
| Secondary Antibody 647 | Thermofischer | Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647, # A-21245 | |
| Slide Scanner | Olympus | VS120 | |
| Snapfrost | Excilone | Excilone Snapfrost | |
| Stab knife | Fine Science Tools | 10316-14 | |
| Suture wire dermal | Ethicon | Vicryl 2-0 | |
| Suture wire dura mater | Ethicon | Mersilk 5-0 | |
| Suture wire for catheter | Ethicon | Vycril 3-0 without needle | |
| Suture wire for lifting dura | Ethicon | Prolene 6-0 with BV-1 needle | |
| Suture wire subcutaneous | Ethicon | Vicryl 4-0 | |
| Titanium bridge | Medtronic | TiMesh 015-2001-4 | Cut out the required size |
| Titanium screws | Medtronic | 9001635, 9001640 | |
| X-ray system | GE | GE OEC 9800 Plus C-Arm |
References
- Ritaccio, A. L., Brunner, P., Schalk, G. Electrical stimulation mapping of the brain: Basic principles and emerging alternatives. Journal of Clinical Neurophysiology. 35 (2), 86-97 (2018).
- Mullin, J. P., Sexton, D., Al-Omar, S., Bingaman, W., Gonzalez-Martinez, J. Outcomes of subdural grid electrode monitoring in the stereoelectroencephalography era. World Neurosurgery. 89, 255-258 (2016).
- Vansteensel, M. J., et al. Fully implanted brain-computer interface in a locked-in patient with ALS. The New England Journal of Medicine. 375 (21), 2060-2066 (2016).
- Lacour, S. P., Courtine, G., Guck, J. Materials and technologies for soft implantable neuroprostheses. Nature Reviews. Materilas. 1, 16063 (2016).
- Fallegger, F., Schiavone, G., Lacour, S. P. Conformable hybrid systems for implantable bioelectronic interfaces. Advanced Materials. 32 (15), 1903904 (2019).
- Pearce, A. I., Richards, R. G., Milz, S., Schneider, E., Pearce, S. G. Animal models for implant biomaterial research in bone: A review. European Cells & Materials. 13, 1-10 (2007).
- Swindle, M. M., Makin, A., Herron, A. J., Clubb, F. J., Frazier, K. S. Swine as models in biomedical research and toxicology testing. Veterinary Pathology. 49 (2), 344-356 (2012).
- Khoshnevis, M., et al. Development of induced glioblastoma by implantation of a human xenograft in Yucatan minipig as a large animal model. Journal of Neuroscience Methods. 282, 61-68 (2017).
- Borton, D., et al. Developing implantable neuroprosthetics: A new model in pig. Annual International Conference of the IEEE Engineering in Medicine & Biology Society. 2011, 3024-3030 (2011).
- Sauleau, P., Lapouble, E., Val-Laillet, D., Malbert, C. H. The pig model in brain imaging and neurosurgery. Animal. 3 (8), 1138-1151 (2009).
- Gierthmuehlen, M., et al. Evaluation of mECoG electrode arrays in the minipig: Experimental procedure and neurosurgical approach. Journal of Neuroscience Methods. 202 (1), 77-86 (2011).
- Palma, M., et al. Chronic recording of cortical activity underlying vocalization in awake minipigs. Journal of Neuroscience Methods. 366, 109427 (2022).
- Bjarkam, C. R., Glud, A. N., Orlowski, D., Sørensen, J. C. H., Palomero-Gallagher, N. The telencephalon of the Göttingen minipig, cytoarchitecture and cortical surface anatomy. Brain Structure & Function. 222 (5), 2093-2114 (2017).
- Lind, N. M., et al. The use of pigs in neuroscience: Modeling brain disorders. Neuroscience and Biobehavioral Reviews. 31 (5), 728-751 (2007).
- Shepherd, R. K., Villalobos, J., Burns, O., Nayagam, D. A. X. The development of neural stimulators: A review of preclinical safety and efficacy studies. Journal of Neural Engineering. 15 (4), 041004 (2018).
- Schiavone, G., et al. Soft, implantable bioelectronic interfaces for translational research. Advanced Matererials. 32 (17), 1906512 (2020).
- Fallegger, F., et al. MRI-compatible and conformal electrocorticography grids for translational research. Advanced Science. 8 (9), 2003761 (2021).
- Minev, I. R., Wenger, N., Courtine, G., Lacour, S. P. Research update: Platinum-elastomer mesocomposite as neural electrode coating. APL Materials. 3 (1), 014701 (2015).
- Schiavone, G., et al. Guidelines to study and develop soft electrode systems for neural stimulation. Neuron. 108 (2), 238-258 (2020).
- Musigazi, G. U., De Vleeschauwer, S., Sciot, R., Verbeken, E., Depreitere, B. Brain perfusion fixation in male pigs using a safer closed system. Laboratory Animals. 52 (4), 413-417 (2018).
- Chung, K., Deisseroth, K. CLARITY for mapping the nervous system. Nature Methods. 10 (6), 508-513 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved