Measuring Tropospheric Ozone
Genel Bakış
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
Ozone is a form of elemental oxygen (O3), a molecule of three oxygen atoms bonded in a structure that is highly reactive as an oxidizing agent. Ozone occurs in both the stratosphere and the troposphere levels of the atmosphere. When in the stratosphere (located approximately 10-50 km from the earth’s surface), ozone molecules form to the ozone layer and help prevent harmful UV rays from reaching Earth’s surface. In lower altitudes of the troposphere (surface - approximately 17 km), ozone is harmful to human health and is considered an air pollutant contributing to photochemical smog (Figure 1). Ozone molecules can cause damage directly by harming respiratory tissue when inhaled or indirectly by harming plant tissues (Figure 2) and softer materials including tires on automobiles.
Outdoor tropospheric ozone is formed at ground level when nitrogen oxides (NOx) and volatile organic compounds (VOCs) from automobile emissions are exposed to sunlight. Consequently, health concerns over ozone concentrations escalate in sunny conditions or when and where automobile use is increased.
Reaction: NO2 + VOC + sunlight → O3 (+ other products)
Indoor tropospheric ozone is formed when electrical discharges from equipment using high voltages (e.g. ionic air purifiers, laser printers, photocopiers) break down the chemical bonds of the atmospheric oxygen (O2) in the air surrounding the equipment:
O2 → 2 O
The free radicals of oxygen in and around electrical discharge recombine to create ozone (O3).
2 O + 2 O2→ 2 O3

Figure 1: Golden Gate Bridge panorama
Characteristic coloration for smog in California in the beige cloud bank behind the Golden Gate Bridge. The brown coloration is due to the NOx in the photochemical smog.

Figure 2: Plants damaged by ozone. Top row is normal, bottom row has been exposed to ozone.
İlkeler
Tropospheric ozone can be monitored by using a mixture of starch, potassium iodide, and water spread on filter paper. Once dried, the paper, called Schönbein paper, changes color when ozone is present.
The method is based on the oxidation capability of ozone. Ozone in the air will oxidize the potassium iodide on the test paper to produce iodine:
2 Kl + O3 + H2O → 2 KOH + O2 + I2
The iodine then reacts with the starch, staining the paper a shade of violet. The intensity of the color depends on the amount of ozone present in the air. The darker the color, the more ozone is present:
I3-+ starch → violet color
Ozone concentration is sampled at different sites of higher risk including parking lots, garages, parkways, and corners of heavily trafficked streets. Indoor sites include room and spaces with equipment involving ink printing, such as copiers.
Prosedür
1. Schönbein Paper Preparation
- Place 100 mL of distilled water in a 250-mL beaker.
- Add 1¼ teaspoon of cornstarch.
- Place a stir bar in the beaker, and place the beaker on a hot/stir plate. Heat on a medium to high setting, and stir the mixture slowly until it gels near approximately 90 °C. The mixture is gelled when it thickens and becomes somewhat translucent.
- Remove the beaker from the heat source, add ¼ teaspoon of potassium iodide, and stir well.
- Cool the solution before applying to the filter paper.
- Lay a piece of filter paper on a glass plate or hold it in the air, and using a small paint brush, carefully brush the paste onto the filter paper. Turn the filter paper over and do the same on the other side. Try to apply the paste as uniformly as possible.
- Set the paper out overnight and away from sunlight, or place in a low temperature (20 °C) drying oven to dry.
- Once the paper is dry, use scissors to cut the filter paper into 1-inch wide strips. If storing the paper for later use, place the strips in a sealable plastic bag or glass jar, out of direct sunlight.
2. Ozone Measurement
- Spray strips of test paper with distilled water and hang a minimum of three strips at each data collection site out of direct sunlight. Hang by securely taping or tacking one end of the strip to a structure (e.g. wall, parking blocks, light poles). Strips can also be hung using wire with one end driven into the ground and the other end secured to the strip. Ensure the strips hang unobstructed.
- Expose the paper for approximately 8 h. Note where each strip was hung, and use weather data to record relative humidity location during paper exposure. Alternatively, a psychrometer can be used to measure relative humidity of each site.
- If the results will not be recorded immediately, seal the strip in an airtight container after exposure.
- To observe and record test results, spray the paper with distilled water.
- Observe the color by comparing it to the provided color scale and recording the corresponding Schönbein number. Calculate the average Schönbein number for each site.
- Use the relative humidity data for each site and the Relative Humidity Schönbein Number Chart (Figure 3) to convert Schönbein site averages to ozone concentration (ppb).
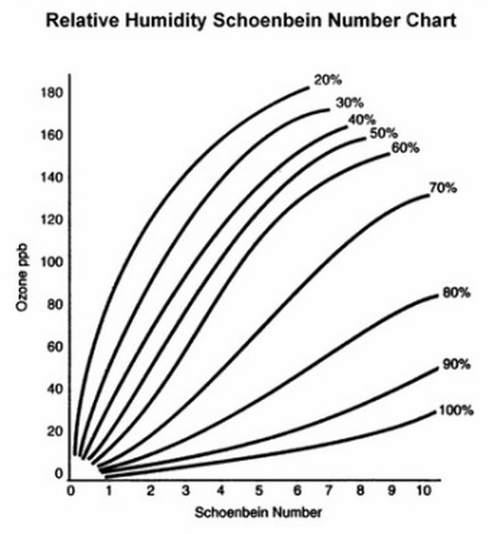
Figure 3. Relative Humidity Schönbein Number Chart
Sonuçlar
Use the Schönbein number scale (Figure 4) for quantitative analysis of ozone. The chart is used to compare with sample papers after 8 h of exposure at sample locations. Use the Relative Humidity Schönbein Number Chart to convert Schönbein scores to ozone concentration (ppb) (Figure 5).
Score increases with increasing color intensity, with the darkest violet on the right side of the scale. Results should vary based on the location of the collection site (Figure 5).
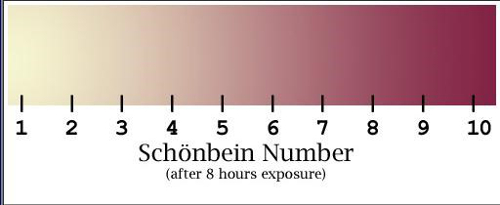
Figure 4. Schönbein Number Scale
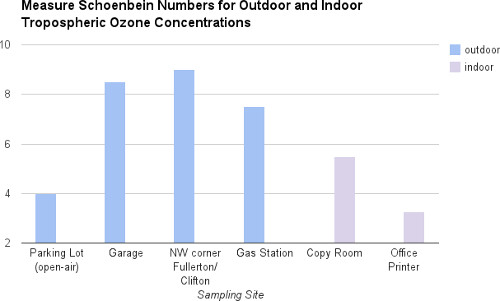
Figure 5. Graph of sample ozone concentrations.
Outdoor and indoor tropospheric ozone concentrations shown by site and Schönbein score.
Başvuru ve Özet
Tropospheric ozone exposure is harmful to human health; known to cause chest pain, coughing, throat irritation, and congestion. Ozone also interferes with lung function, exacerbating symptoms of bronchitis, emphysema, and asthma, and can permanently damage lung tissue.
Outdoor locations of increased amounts of sunlight and urban areas experience higher levels of tropospheric ozone due to increased amount and density of nitrate emissions. Indoor locations where copy machines and ink printers are used are also high-risk areas for ozone exposure. Current US thresholds for ozone, set by the Office of Safety and Health, is 0.1 ppm with health risks including headache, irritation to eyes, nose and throat, brain and nervous system damage, lung damage, chronic respiratory disease, pulmonary congestion, edema, and hemorrhage.
Atla...
Bu koleksiyondaki videolar:

Now Playing
Measuring Tropospheric Ozone
Environmental Science
26.5K Görüntüleme Sayısı

Tree Identification: How To Use a Dichotomous Key
Environmental Science
81.3K Görüntüleme Sayısı

Tree Survey: Point-Centered Quarter Sampling Method
Environmental Science
49.4K Görüntüleme Sayısı

Using GIS to Investigate Urban Forestry
Environmental Science
12.7K Görüntüleme Sayısı

Proton Exchange Membrane Fuel Cells
Environmental Science
22.1K Görüntüleme Sayısı

Biofuels: Producing Ethanol from Cellulosic Material
Environmental Science
53.3K Görüntüleme Sayısı

Testing For Genetically Modified Foods
Environmental Science
89.8K Görüntüleme Sayısı

Turbidity and Total Solids in Surface Water
Environmental Science
35.9K Görüntüleme Sayısı

Dissolved Oxygen in Surface Water
Environmental Science
55.9K Görüntüleme Sayısı

Nutrients in Aquatic Ecosystems
Environmental Science
38.9K Görüntüleme Sayısı

Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Environmental Science
30.2K Görüntüleme Sayısı

Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Environmental Science
125.6K Görüntüleme Sayısı

Carbon and Nitrogen Analysis of Environmental Samples
Environmental Science
29.5K Görüntüleme Sayısı

Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Environmental Science
216.0K Görüntüleme Sayısı

Analysis of Earthworm Populations in Soil
Environmental Science
16.5K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır