A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Adhesion of Candida parapsilosis to Bovine Serum Albumin under Fluid Shear
In This Article
Summary
Adhesion is an important first step in colonization and pathogenesis for Candida. Here, an in vitro assay is described to measure adhesion of C. parapsilosis isolates to immobilized proteins under fluid shear. A multichannel microfluidics device is used to compare multiple samples in parallel, followed by quantification using fluorescence imaging.
Abstract
C. parapsilosis (Cp) is an emerging cause of bloodstream infections in certain populations. The Candida clade, including Cp, is increasingly developing resistance to the first and the second line of antifungals. Cp is frequently isolated from hands and skin surfaces, as well as the GI tract. Colonization by Candida predisposes individuals to invasive bloodstream infections. To successfully colonize or invade the host, yeast must be able to rapidly adhere to the body surfaces to prevent elimination by host defense mechanisms. Here we describe a method to measure adhesion of Cp to immobilized proteins under physiologic fluid shear, using an end-point adhesion assay in a commercially available multichannel microfluidic device. This method is optimized to improve reproducibility, minimize subjectivity, and allow for the fluorescent quantification of individual isolates. We also demonstrate that some clinical isolates of Cp show increased adhesion when grown in conditions mimicking a mammalian host, whereas a frequently used lab strain, CDC317, is non-adhesive under fluid shear.
Introduction
Candida spp. are common commensal organisms on human skin and mucosae that can lead to invasive diseases among the immunocompromised with substantial associated morbidity, mortality, and cost1,2,3. Although C. albicans remains an important cause of these infections, non-albicans species such as C. parapsilosis, C. glabrata, C. krusei, C. tropicalis, and C. auris are being increasingly recognized, especially in vulnerable populations and with frequent resistance to available antifungal drugs4. Non-albicans species present distinct elements of biology and pathogenesis that are under active investigation.
Adhesion is an important first step in colonization and pathogenesis. Interference with this step may therefore offer an opportunity to stop disease progression at an early stage. Studies of Candida adhesion and invasion have been predominantly focused on static conditions5,6. These studies have helped define the structure and functions of fungal adhesins in disease7,8,9. However, adhesion in the bloodstream, gastro-intestinal (GI) tracts, and urinary tracts, and in catheters must occur under conditions of fluid shear flow which places unique constraints upon adhesion. Adhesion under shear requires rapid catch bond formation and the ability to withstand strong pulling forces produced due to the movement of liquids10,11. The C. albicans adhesin, Als5 has been shown to facilitate shear dependent adhesion12,13. CpAls7 (CpALS4800) has been previously shown to mediate adhesion of Cp to epithelial cells, and a knockout showed decreased virulence in a urinary tract infection model14. We demonstrated that CpALS4800 promotes adhesion under physiologically relevant fluid shear conditions15.
Candida colonization and pathogenesis have been extensively studied in the animal models16,17,18. The most frequently used models are murine mucosal and bloodstream infections but invertebrate models, such as Galleria larvae, are increasingly being used because of the low cost, rapid throughput, and simplicity. Animal models recapitulate many steps of the human disease process in both the pathogen and host, including the host adaptive and innate immune responses, interactions of yeast with tissues and the microbiota, and yeast responses to the host environment. In contrast, in vitro adhesion assays permit the focus specifically on the adhesion step, and on the experimental manipulation of variables such as shear force, growth conditions of yeast, and adhesion to specific substrates.
Because Cp is capable of growth in both humans and environmental sources, it is likely to be capable of sensing and responding to different environments. In support of this notion, multiple clinical isolates of Cp show low adhesion under fluid shear when grown in the standard yeast growth medium, yeast-peptone-dextrose (YPD), but switch to strong adhesion when grown for a few hours at 37 °C in the tissue-culture medium 199 (M199)15,19. A detailed protocol is provided here for a medium throughput assay that permits the measurement of adhesion of multiple yeast samples that run in parallel, under defined conditions of growth, fluid shear, temperature, and substrate. The assay has been designed to maximize reproducibility, and to allow for the use of clinical isolates of Cp, as well as strains that have been experimentally manipulated in the lab. The assay as described here, for Cp adhesion to a bovine serum albumin (BSA) substrate, demonstrates that clinical isolates exhibit a range of adhesion, whereas two commonly used lab strains, CDC317 and CLIB214 show poor adhesion.
Protocol
Candida spp. are classified as Biosafety Level 2 organisms and should be handled using appropriate precautions.
1. Growth and induction of clinical strains
- Streak Cp strains on 1% (m/v) yeast extract, 2% (m/v) peptone, 2% (m/v) dextrose (YPD) 2% (m/v) agar plates, and grow at 22 °C.
NOTE: Plates may be stored on the lab bench and re-used over the following week. - The day prior to the adhesion assay, transfer approximately 6 colonies of each strain to a 250 mL Erlenmeyer (conical) flask containing 10 mL of YPD medium. Grow overnight in a microbiological shaker set to 250 rpm at 37 °C.
NOTE: Cp need to grow for 20-24 h to reach the stationary phase in liquid culture at 37 °C. - Perform the following steps on the day of the adhesion assay.
- Transfer 1 mL of the liquid culture from the Erlenmeyer flask to a microfuge tube.
- Centrifuge the culture at a maximum speed in a microfuge (16,000 x g) for 3 min. Resuspend the pellet in 1 mL of sterile water and repeat this step for a total of three washes.
- At the end of the last wash, resuspend the pellet in 1 mL of sterile water. Use wide-orifice pipette tips of 1 mL and 200 µL sizes for handling yeast suspensions during this and subsequent steps.
NOTE: Many adhesive strains of Cp stick to the sides of the Erlenmeyer flask, and require mechanical scraping to remove.
- Count yeast cells using a hemocytometer or equivalent device. Dilute the yeast culture to achieve a reasonably countable concentration of 50-200 yeast cells. Use a 500-fold dilution, putting 20 µL into 10 mL of water.
NOTE: Most Cp strains grow to 108-109 cells/mL in an overnight shaking culture at 37 °C. - Dilute the yeast culture with 2 mL of YPD or Medium 199 (M199), at a final concentration of 3 x 106 cells/mL in a 2 mL microfuge tube. Incubate in a 37 °C water bath for 3 h.
2. Coating of microfluidic channels
- Prepare in advance a 2% (m/v) solution of BSA in Hank's Balanced Salt Solution containing calcium and magnesium (HBSS+). To do this, gently sprinkle 4 g of BSA powder on the surface of 200 mL of HBSS+ and incubate at 37 °C for 30-60 min undisturbed to allow the protein to wet and then dissolve. Filter-sterilize the solution and store at 4 °C.
- Warm 2.5 mL of this solution to 37 °C for at least 1 h, keeping it sterile.
NOTE: Warming is necessary to reduce the bubble formation in the microchannel plate. - While the BSA is warming, move the microfluidics controller to the tissue culture hood, turn on the device and start the software.
- Place the fluidics interface within the sterile field, and gently clean the silicone gasket with a lint-free tissue paper wetted with 70% (v/v) alcohol. Avoid prolonged or repeated contact of alcohol with the acrylic plate to prevent crazing or cracking of the plastic.
- Air dry the interface (facing up) in the hood with an airflow system to remove all traces of alcohol.
- Add 100 µL of the pre-warmed BSA solution as a droplet in the central indentation of each of the "outlet" channel (Figure 1A) of a 48 well, 24-microchannel plate.
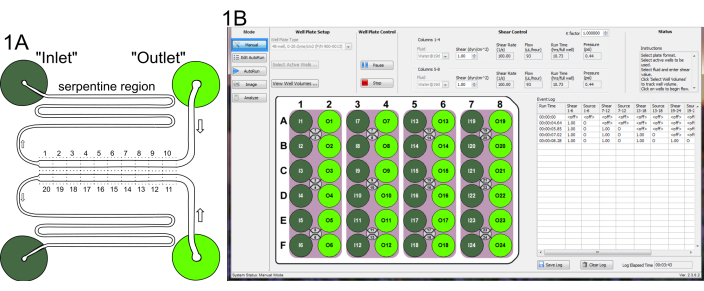
Figure 1. Microfluidics assay layout. (A) A pair of channels, showing reverse fluid flow from the "outlet" to the "inlet". The consecutive tiled fields captured by the microscope are shown by dotted lines (1-10 for the upper channel, and 11-20 for the lower). (B) Setup of microfluidics controller software for reverse flow during BSA coating (Step 2.10). Screenshots reproduced here with permission from the manufacturer. Please click here to view a larger version of this figure.
- Place the interface on the top of the microchannel plate, aligning the four bolts of the interface with their corresponding sockets in the plate.
- Tighten the bolts using gloved fingers. Be aware that the resistance to hand tightening indicates misalignment. If this occurs, lift the interface slightly and reseat it, and resume fastening the bolts.
- When the bolts are finger tight, use the torque wrench to further tighten the interface.
- Using the microfluidics software interface (Figure 1B), set the Mode to Manual. Use the default Fluid option (Water@19degC) for both sets of columns and set shear to 1 dyn/cm^2.
- Activate "outlet" columns (#2,4,6,8) to pump liquid towards "inlets". Run at room temperature for 30 min.
- Visually inspect each "inlet" well by peering through the bottom of the microchannel plate to ensure that a droplet of the BSA solution has pooled in each "inlet". This confirms that all 24 channels were successfully wetted and filled.
- Unfasten the interface and top up each well ("inlets" and "outlets") with 250 µL of HBSS+ without BSA to prevent drying out of channels and put the plate in a tissue culture incubator.
NOTE: A minimum of 48 h is required for proteins to be uniformly adsorbed to the channel surfaces of the microchannel plate. After protein coating, plates may be stored for up to two weeks in a humid environment (such as a tissue culture incubator with 100% saturating humidity) before the use in adhesion assays. For longer periods, wrap plates in plastic film to prevent evaporation.
3. Adhesion assay
- During yeast incubation in YPD and M199 (step 1.5), aspirate inlet and outlet wells of the BSA coated microchannel plate, without disturbing the channel that runs from the central indentation of each well (Figure 1A). Instead, aspirate from the edge of the well. Add 1 mL of HBSS+ to the "outlet" wells.
- Attach the microfluidics interface as above (step 2.5). Use the default fluid setting Fluid at (Water@19degC) and Shear at 2 dyn/cm^2 at room temperature to wash the channel and remove any unbound BSA. Wash for 2-3 h at this flow rate.
- Remove the interface from the microchannel plate. Turn on the plate heater unit of the microfluidics device and confirm that it is set to 37 °C.
- Aspirate the medium from all wells on the plate and add 0.5 mL of induced yeast from step 1.5 to each pair of "outlets". Resuspend the yeast completely by inverting the 2 mL tubes 3-6 times, and gently pipette up and down immediately prior to the addition to wells. Leave the "inlet" wells empty.
- Fasten fluidics interface to the microchannel plate as in step 2.5 above. Use device software to set Fluid to HBSS@37degC, and Shear to 5 dyn/cm^2.
- Activate "outlet" columns (#2,4,6,8) to pump liquid towards "inlets". Run on the plate heater at 37 °C for 30 min, to allow yeast to adhere to the BSA-coated channel.
- During this time, prepare the wash buffer. To 30 mL of Dulbecco's phosphate-buffered saline containing calcium and magnesium (DPBS+) add 5 µM of calcofluor, which is included to render the yeast fluorescent for their detection. Warm in a bath at 37 °C.
- At the end of the 30 min adhesion, Pause the flow using the software interface without altering other flow conditions. Unfasten the interface and aspirate all wells ("inlets" and "outlets").
- Add 1 mL of calcofluor wash buffer to "outlets". Reattach fluidics interface, and resume the flow using the software for another 10 min. This step is designed to wash away non-adherent and loosely bound yeast, and simultaneously fluorescently stain yeast in the channel.
- After 10 min, remove the fluidics interface and replace with a lid. Gently clean the bottom of the microchannel plate with a lint-free wipe and proceed to imaging.
4. Imaging and quantification
- Place the microchannel plate in an appropriate microscope stage holder. Use a 20x objective lens, which will allow a field of view such that the channel fills approximately half of the image height. Locate the left end of channel 1 (in "inlet" 1). Adjust the stage so that the channel is positioned as in Figure 1A, position 1.
- Acquire a single brightfield image of the channel. Measure the area of the channel area in µm2, using the rectangle measuring tool in order to normalize area measurements during data analysis (see Discussion).
- Switching to the DAPI fluorescent channel (excitation 395 nm, emission 440/40 nm) adjust the focus to adherent yeast on the bottom surface of the channel. Lock the autofocus at this plane. Set the fluorescence excitation intensity to 1.5% and camera exposure conditions (binning 2x2, 15 ms exposure, 12-bit, gain 4) to avoid saturation of the image sensor.
- Using the motorized stage and ND Acquisition | XY Imaging in the microscope controller software, automatically capture a consecutive series of non-overlapping images spaced one field of view apart (666 µm) of first channel pair (1/2).
- Collect 10 images from the left to the right for the upper channel, shift down 666 µm and collect another 10 from the right to the left for the lower channel (as shown schematically in Figure 1A).
- Use the Relative XY option, so that once image positions are defined, a similar series can be triggered for each channel pair, after the start of the channel is manually defined.
- Collect images in the DAPI channel (ND Acquisition | λ | DAPI).
- Monitor images to confirm that the channel remains within the field of view as the motorized stage moves the plate.
NOTE: Each set of 20 images from a channel pair will be automatically saved with a consecutive file name by ND acquisition. - Use the autostep function (XYZ Navigation | XY step) to move 25750 µm down to "inlet" 3. Fine tune channel position manually as in step 4.1. Capture the next set of images (ND Acquisition) of the channel pair 3/4.
- Repeat this process, until all 12 channel pairs have been imaged, with results saved in 12 files. Follow the manufacturer's ordering of channels from 1-24.
- Merge all 12 sets of fluorescent images into a single file (File | Merge ND Documents). Confirm that the file order matches the order in which the 12 sets of images were captured.
- Use Binary | Threshold to separate yeast from the background based on their fluorescence level. Apply the same threshold to the entire stack of images.
NOTE: A 12-bit greyscale intensity low threshold of 500, and a high threshold set to the maximum of 4095 is typical. - Measure the threshold area (Measure | Perform Measurement | All Frames). Open the report (Analysis Controls | Automated Measurement Results) and check data.
NOTE: This will generate a table of measurements for 240 images (20 images from each of 12 channel pairs), and threshold area for each image is listed in the column labeled BinaryArea [µm2]. - Export data to a tab delimited text file (Export).
Results
Using the methods described in the Protocol section, adhesion of 6 strains of Cp was compared (Table 1)
| Strain | Description | Reference/Source |
| JMB81 | Invasive clinical isolate from infant blood culture | 30 |
| JMB77 | Invasive clinical isolate from infant blood culture | 30 |
Discussion
The data resulting from the above protocol can be analyzed using a standard spreadsheet software. Data are expressed as "adhesion index", which is calculated as follows: The BinaryArea value for each set of 10 images (representing the yeast coverage for a single channel) is summed across the images, and the mean and standard deviation are calculated for the summed area of each channel pair. The channel area measured in step 4.2 represents the maximum possible area in a single field of view that might ever be cove...
Disclosures
No disclosures.
Acknowledgements
This work was supported by a grant from the William and Mary Oh-William and Elsa Zopfi Professorship in Pediatrics for Perinatal Research, the Kilguss Research Core, and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P30GM114750.
Materials
| Name | Company | Catalog Number | Comments |
| Bioflux 200 | Fluxion | Bioflux 200 | |
| Bioflux Microfluidics plates, 48 well, low shear | Fluxion | 910-0004 | |
| Bovine Serum Albumin (BSA) Fraction V | Fisher Scientific | BP1605 | |
| Calcofluor Fluorescent Brightener | Sigma-Aldrich | F3543 | |
| DAPI filter set 440/40 | Nikon | ||
| Dulbecco’s Phosphate-Buffered Saline (DPBS+) | Corning Cellgro | 21-030-CM | With calcium and magnesium |
| Hank’s Balanced Salt Solution, 1X (HBSS+) | Corning Cellgro | 21-023-CM | With calcium and magnesium, without phenol red |
| Inverted microscope with Perfect Focus | Nikon | Ti-E | |
| M199 medium | Lonza | 12-117Q | With Earle's salts and HEPES |
| Motorized Stage | Nikon | Ti-S-E | |
| Nikon 20x lambda Plan-Apo objective | Nikon | ||
| NIS-Elements software 5.02 | Nikon | ||
| Spectra fluorescent LED light source | Lumencor | SPECTRA-X3 | |
| Zyla 4.2 sCMOS camera | Andor | Zyla 4.2 |
References
- Pittet, D., Monod, M., Suter, P. M., Frenk, E., Auckenthaler, R. Candida colonization and subsequent infections in critically ill surgical patients. Annals of Surgery. 220 (6), 751-758 (1994).
- Lau, A. F., et al. Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical intensive care units: development and evaluation of a simple, standard protocol. Journal of Clinical Microbiology. 53 (4), 1324-1330 (2015).
- Lionakis, M. S. New insights into innate immune control of systemic candidiasis. Medical Mycology. 52 (6), 555-564 (2014).
- Maubon, D., Garnaud, C., Calandra, T., Sanglard, D., Cornet, M. Resistance of Candida spp. to antifungal drugs in the ICU: Where are we now. Intensive Care Medicine. 40 (9), 1241-1255 (2014).
- Sheppard, D. C., et al. Functional and structural diversity in the Als protein family of Candida albicans. Journal of Biological Chemistry. 279 (29), 30480-30489 (2004).
- Liu, Y., Filler, S. G. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryotic Cell. 10 (2), 168-173 (2011).
- Filler, S. G. Can host receptors for fungi be targeted for treatment of fungal infections. Trends in Microbiology. 21 (8), 389-396 (2013).
- Oh, S. H., et al. Agglutinin-Like Sequence (ALS) genes in the Candida parapsilosis species complex: Blurring the boundaries between gene families that encode cell-wall proteins. Frontiers in Microbiology. 10, 781 (2019).
- Willaert, R. G. Adhesins of yeasts: Protein structure and interactions. Journal of Fungi (Basel). 4 (4), 119 (2018).
- Isberg, R. R., Barnes, P. Dancing with the host; flow-dependent bacterial adhesion. Cell. 110 (1), 1-4 (2002).
- Thomas, W. Catch bonds in adhesion. Annual Review of Biomedical Engineering. 10, 39-57 (2008).
- Chan, C. X., Lipke, P. N. Role of force-sensitive amyloid-like interactions in fungal catch bonding and biofilms. Eukaryotic Cell. 13 (9), 1136-1142 (2014).
- Lipke, P. N., Klotz, S. A., Dufrene, Y. F., Jackson, D. N., Garcia-Sherman, M. C. Amyloid-like beta-aggregates as force-sensitive switches in fungal biofilms and infections. Microbiology and Molecular Biology Reviews. 82 (1), 00035 (2018).
- Bertini, A., et al. Targeted gene disruption in Candida parapsilosis demonstrates a role for CPAR2_404800 in adhesion to a biotic surface and in a murine model of ascending urinary tract infection. Virulence. 7 (2), 85-97 (2016).
- Neale, M. N., et al. Role of the inducible adhesin CpAls7 in binding of Candida parapsilosis to the extracellular matrix under fluid shear. Infections and Immunity. 86 (4), 00892 (2018).
- Clancy, C. J., Cheng, S., Nguyen, M. H. Animal models of candidiasis. Methods in Molecular Biology. 499, 65-76 (2009).
- MacCallum, D. M. Mouse model of invasive fungal infection. Methods in Molecular Biology. 1031, 145-153 (2013).
- Segal, E., Frenkel, M. Experimental in vivo models of candidiasis. Journal of Fungi (Basel). 4 (1), 21 (2018).
- Bliss, J. M., et al. Transcription profiles associated with inducible adhesion in Candida parapsilosis. mSphere. 6 (1), 01071 (2021).
- Hochmuth, R. M., et al. Surface adhesion, deformation and detachment at low shear of red cells and white cells. Transactions - American Society for Artificial Internal Organs. 18, 325-334 (1972).
- Munn, L. L., Melder, R. J., Jain, R. K. Analysis of cell flux in the parallel plate flow chamber: implications for cell capture studies. Biophysical Journal. 67 (2), 889-895 (1994).
- Shaw, S. K., Bamba, P. S., Perkins, B. N., Luscinskas, F. W. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. Journal of Immunology. 167 (4), 2323-2330 (2001).
- Shaw, S. K., et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. Journal of Experimental Medicine. 200 (12), 1571-1580 (2004).
- Grubb, S. E., et al. Adhesion of Candida albicans to endothelial cells under physiological conditions of flow. Infection and Immunity. 77 (9), 3872-3878 (2009).
- Finkel, J. S., et al. Portrait of Candida albicans adherence regulators. PLoS Pathogens. 8 (2), 1002525 (2012).
- Gulati, M., Ennis, C. L., Rodriguez, D. L., Nobile, C. J. Visualization of biofilm formation in Candida albicans using an automated microfluidic device. Journal of Visualized Experiments. (130), e56743 (2017).
- Shaik, S. S. Low intensity shear stress increases endothelial ELR+ CXC chemokine production via a focal adhesion kinase-p38β MAPK-NF-κβ pathway. Journal of Biological Chemistry. 284 (9), 5945-5955 (2009).
- dela Paz, N. G., Walshe, T. E., Leach, L. L., Saint-Geniez, M., D'Amore, P. A. Role of shear-stress-induced VEGF expression in endothelial cell survival. Journal of Cell Science. 125, 831-843 (2012).
- Bliss, J. M., Basavegowda, K. P., Watson, W. J., Sheikh, A. U., Ryan, R. M. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatric Infectious Disease Journal. 27 (3), 231-235 (2008).
- Bliss, J. M., et al. Candida virulence properties and adverse clinical outcomes in neonatal candidiasis. Journal of Pediatrics. 161 (3), 441-447 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved