Proton Exchange Membrane Fuel Cells
Overview
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
The United States consumes a large amount of energy – the current rate is around 97.5 quadrillion BTUs annually. The vast majority (90%) of this energy comes from non-renewable fuel sources. This energy is used for electricity (39%), transportation (28%), industry (22%), and residential/commercial use (11%). As the world has a limited supply of these non-renewable sources, the United States (among others) is expanding the use of renewable energy sources to meet future energy needs. One of these sources is hydrogen.
Hydrogen is considered a potential renewable fuel source, because it meets many important criteria: it’s available domestically, it has few harmful pollutants, it’s energy efficient, and it’s easy to harness. While hydrogen is the most abundant element in the universe, it is only found in compound form on Earth. For example, it is combined with oxygen in water as H2O. To be useful as a fuel, it needs to be in the form of H2 gas. Therefore, if hydrogen is to be used as a fuel for cars or other electronics, H2 needs to be made first. Thusly, hydrogen is often called an “energy carrier” rather than a “fuel.”
Currently, the most popular way to make H2 gas is from fossil fuels, through steam reforming of hydrocarbons or coal gasification. This does not reduce dependence on fossil fuels and is energy intensive. A less-used method is by electrolysis of water. This also requires an energy source, but it can be a renewable source, like wind or solar power. In electrolysis, water (H2O) is split into its component parts, hydrogen gas (H2) and oxygen gas (O2), through an electrochemical reaction. The hydrogen gas made through the process of electrolysis can then be used in a Proton Exchange Membrane (PEM) fuel cell, generating an electric current. This electric current can be used to power motors, lights, and other electrical devices.
Procedure
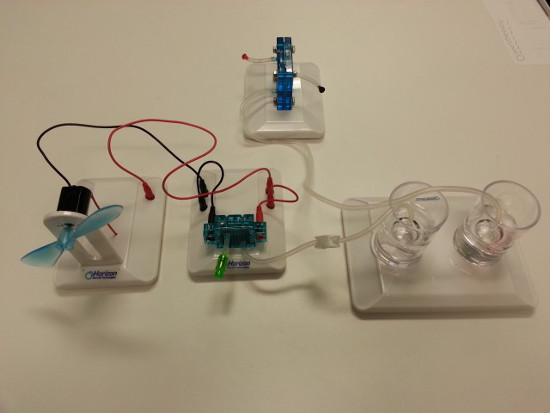
1. Using the Electrolyzer to Produce Hydrogen Gas
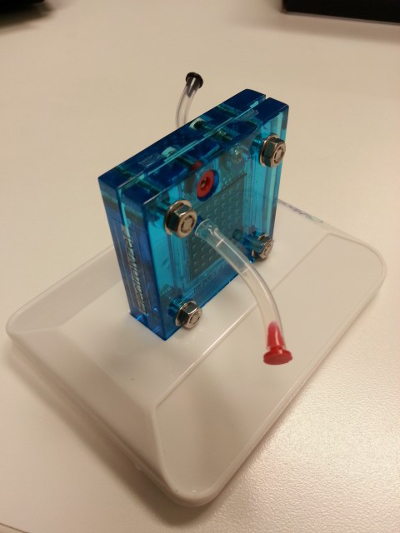
- Setup the electrolyzer (Figure 3).
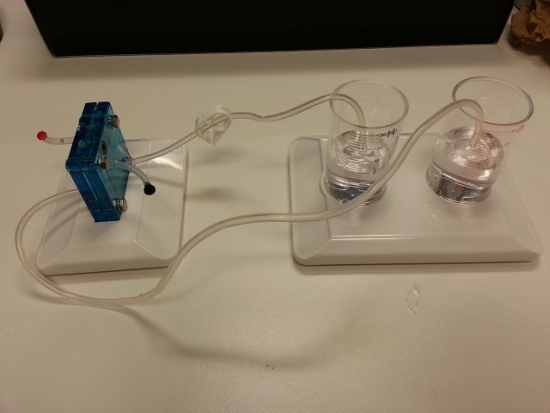
- Set up the gas collection cylinders, making sure the distilled water level in the outer cylinder is at the 0 mark (Figure 4).
- Connect the electrolyzer to the gas collection cylinders (Figure 5).
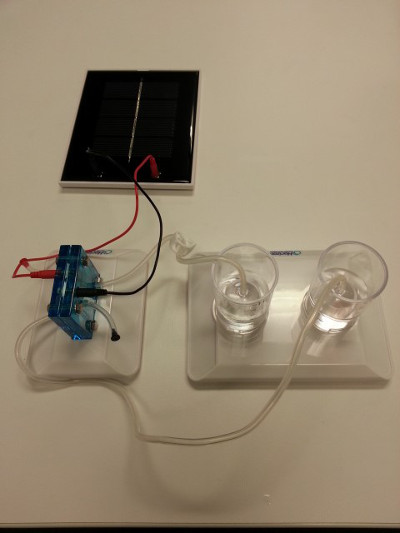
- Connect a solar panel to the electrolyzer using jumper wires and expose to direct sunlight (Figure 6). Note, if the weather is not coope
Results
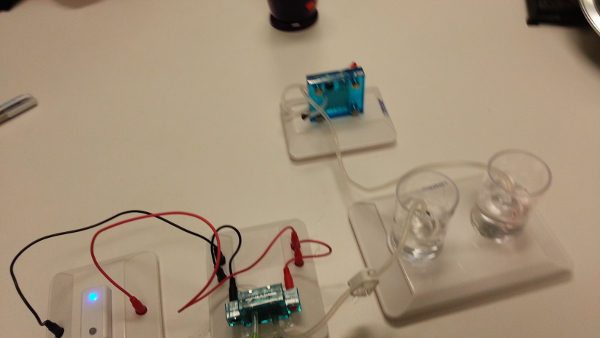
During the electrolysis procedure, hydrogen and oxygen gas are generated once the solar panel is connected and exposed to sunlight. It takes approximately 10 min to generate enough H2 gas to fill the inner cylinder (Table 1). Note that there is twice as much H2 generated as O2, as seen in the balanced equation:
2 H2O(l) → 2 H2(g) + O2(g)
Onc
Application and Summary
Hydrogen is a flexible fuel. It can be produced on-site in small quantities for local use or in large quantities at a centralized facility. The hydrogen can then be used to produce electricity with only water as a byproduct (provided a renewable source of energy, like a wind turbine, was used to generate the hydrogen gas). For example, in Boulder, Colorado, the Wind2H2 project has wind turbines and solar panels connected to electrolyzers that produce hydrogen gas from water and then stores it to be used in their hydrogen
Skip to...
Videos from this collection:

Now Playing
Proton Exchange Membrane Fuel Cells
Environmental Science
22.2K Views

Tree Identification: How To Use a Dichotomous Key
Environmental Science
81.4K Views

Tree Survey: Point-Centered Quarter Sampling Method
Environmental Science
49.5K Views

Using GIS to Investigate Urban Forestry
Environmental Science
12.7K Views

Biofuels: Producing Ethanol from Cellulosic Material
Environmental Science
53.4K Views

Testing For Genetically Modified Foods
Environmental Science
90.1K Views

Turbidity and Total Solids in Surface Water
Environmental Science
35.9K Views

Dissolved Oxygen in Surface Water
Environmental Science
56.0K Views

Nutrients in Aquatic Ecosystems
Environmental Science
39.0K Views

Measuring Tropospheric Ozone
Environmental Science
26.5K Views

Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Environmental Science
30.3K Views

Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Environmental Science
125.9K Views

Carbon and Nitrogen Analysis of Environmental Samples
Environmental Science
29.6K Views

Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Environmental Science
216.2K Views

Analysis of Earthworm Populations in Soil
Environmental Science
16.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved