Organocatalysis
Overview
Source: Vy M. Dong and Faben Cruz, Department of Chemistry, University of California, Irvine, CA
This experiment will demonstrate the concept of organocatalysis by illustrating the proper setup of a reaction that utilizes enamine catalysis. Organocatalysis is a form of catalysis that uses substoichiometric amounts of small organic molecules to accelerate reactions. This type of catalysis is complementary to other forms of catalysis such as transition metal or biocatalysis. Transition metal catalysis involves transition metals as catalysts and biocatalysis uses enzymes as catalysts. Some advantages of organocatalysis include the low toxicity and cost of the organocatalysts in comparison to many metal catalysts. In addition, most organocatalysts are not sensitive to air and moisture, unlike metal catalysts. In contrast to enzymes found in living organisms, the small molecules that act as organocatalysts are typically easy to access. Furthermore, organocatalysis offers complementary and new reactivity not observed with other forms of catalysis.
Principles
Organocatalysts can be divided into four categories based on the type of catalyst. Most organocatalysts can be described as Lewis bases, Lewis acids, Bronsted bases, or Bronsted acids. These organocatalyst categories describe the mode of activation by which the catalyst acts to facilitate catalysis. In addition to these different modes of activation, organocatalysts can interact with substrates via covalent or non-covalent interactions; both of which have their advantages and disadvantages. Typically, covalent interactions are easier to control and thus predict. Oftentimes, catalysts that take advantage of non-covalent interactions require lower catalyst loadings in comparison to those that operate via covalent interactions.
Lewis bases, especially amines, are the most common type of organocatalyst. Several types of reactivity have been achieved by only using an amine catalyst. For example, the nucleophilicity of nucleophiles can be accentuated via enamine catalysis to perform selective alkylations or aldol reactions. Amine-based catalysts can also improve the electrophilicity of substrates via iminium catalysis to promote Michael additions or cycloadditions. Amine-based catalysts can even be used as phase-transfer catalysts to mediate reactions between two media phases.
In addition to substrate activation, these catalysts can also introduce chirality into the products they form, in a concept called asymmetric catalysis. One of the first examples of asymmetric organocatalysis used a chiral amino acid, proline, to catalyze an Aldol reaction (Figure 1). Proline condenses onto one of the ketones to generate a chiral enamine. In doing so, the organocatalyst generates a stronger nucleophile and introduces chirality such that the Aldol reaction can be stereoselective. The depicted example is of the Hajos-Parrish-Eder-Sauer-Wiechert reaction. The product of this reaction is an important precursor for the synthesis of steroid natural products and their derivatives.
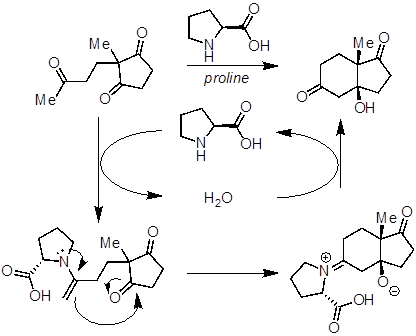
Figure 1: One of the first examples of asymmetric organocatalysis used a chiral amino acid, proline, to catalyze an Aldol reaction.
Procedure

- Add (S)-proline (40 mg, 0.35 mmol, 0.35 equivalents), acetonitrile (MeCN, 5 mL), and the diketone (126 mg, 1 mmol, 1 equivalent) to a round-bottom flask (~ 20 mL) equipped with a magnetic stir bar.
- Stir the reaction mixture at 35 °C for 30 min.
- Add 3-buten-2-one (105 mg, 1.5 mmol, 1.5 equivalents) dropwise at 35 °C and stir at the same temperature for 1 week.
- Cool the reaction to room temperature and quench by adding ~ 5 mL of saturated aqueous ammonium chloride.
- Extract the aqueous layer with diethyl ether.
- Wash the combined organic layers with brine and dry with anhydrous magnesium sulfate.
- Filter the magnesium sulfate and concentrate via rotatory evaporation.
- Purify the crude residue via column chromatography.
Results
The purified product should have the following 1H NMR spectrum: 1H NMR δ 5.88 (1H, s), 2.6-2.7 (2H, m), 2.3-2.55 (4H, m), 2.0-2.2 (2H, m), 1.6-1.8 (2H, m), 1.4 (3H, s).
Application and Summary
This experiment has demonstrated how to set up an enamine catalyzed reaction. Compared to other forms of catalysis, organocatalysis is a relatively young field of research, but in recent years the field of organocatalysis has experienced dramatic growth. The increased interest in organocatalysis has also given rise to research that makes use of more than one type of catalysis to achieve new types of reactivity. For example, there has been increased reports of using organocatalysis in conjunction with transition metal catalysis.
Asymmetric organocatalysis has been used to improve the synthesis of warfarin, a common anti-coagulant. The previous synthetic route relied upon chemical resolution (an inherently wasteful process) of the racemic mixture to afford the more active enantiomer (S)-warfarin in 19% yield. Now with the aid of asymmetric organocatalysis, (S)-warfarin can now be accessed without chemical resolution in 99% yield via iminium catalysis.

Figure 2: (S)-Warfarin.
The antiviral medication, Tamiflu, that is used to treat the flu has been synthesized using organocatalysis. This synthesis makes use of a common type of organocatalyst, a prolinol-derived catalyst. The organocatalyzed Michael addition sets two out of the three necessary stereocenters found in Tamiflu.

Figure 3: The antiviral medication, Tamiflu.
Tags
Skip to...
Videos from this collection:

Now Playing
Organocatalysis
Organic Chemistry II
17.0K Views

Cleaning Glassware
Organic Chemistry II
123.9K Views

Nucleophilic Substitution
Organic Chemistry II
99.6K Views

Reducing Agents
Organic Chemistry II
43.2K Views

Grignard Reaction
Organic Chemistry II
149.3K Views

n-Butyllithium Titration
Organic Chemistry II
48.1K Views

Dean-Stark Trap
Organic Chemistry II
100.7K Views

Ozonolysis of Alkenes
Organic Chemistry II
67.2K Views

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.7K Views

Solid Phase Synthesis
Organic Chemistry II
41.3K Views

Hydrogenation
Organic Chemistry II
49.7K Views

Polymerization
Organic Chemistry II
94.9K Views

Melting Point
Organic Chemistry II
150.1K Views

Infrared Spectroscopy
Organic Chemistry II
216.2K Views

Polarimeter
Organic Chemistry II
100.3K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved